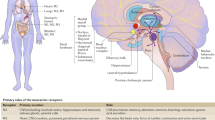
Abstract
Overactive bladder (OAB) is a common condition, particularly in the elderly. Anticholinergic agents are the mainstay of pharmacological treatment of OAB; however, many anticholinergics can cross the blood-brain barrier (BBB) and may cause central nervous system (CNS) effects, including cognitive deficits, which can be especially detrimental in older patients. Many anticholinergics have the potential to cause adverse CNS effects due to muscarinic (M1) receptor binding in the brain. Of note, permeability of the BBB increases with age and can also be affected by trauma, stress, and some diseases and medications. Passive crossing of a molecule across the BBB into the brain is dependent upon its physicochemical properties. Molecular characteristics that hinder passive BBB penetration include a large molecular size, positive or negative ionic charge at physiological pH, and a hydrophilic structure. Active transport across the BBB is dependent upon protein-mediated transporter systems, such as that of permeability-glycoprotein (P-gp), which occurs only for P-gp substrates, such as trospium chloride, darifenacin and fesoterodine. Reliance on active transport can be problematic since genetic polymorphisms of P-gp exist, and many commonly used drugs and even some foods are P-gp inhibitors or are substrates themselves and, due to competition, can reduce the amount of the drug that is actively transported out of the CNS. Therefore, for drugs that are preferred not to cross into the CNS, such as potent anticholinergics intended for the bladder, it is optimal to have minimal passive crossing of the BBB, although it may also be beneficial for the drug to be a substrate for an active efflux transport system. Anticholinergics demonstrate different propensities to cross the BBB. Darifenacin, fesoterodine and trospium chloride are substrates for P-gp and, therefore, are actively transported away from the brain. In addition, trospium chloride has not been detected in cerebrospinal fluid assays and does not appear to have significant CNS penetration. This article reviews the properties of anticholinergics that affect BBB penetration and active transport out of the CNS, discusses issues of increased BBB permeability in patients with OAB, and examines the clinical implications of BBB penetration on adverse events associated with anticholinergics.




Similar content being viewed by others
References
Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol 2003 May; 20(6): 327–36
Irwin DE, Milsom I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol 2006 Dec; 50(6): 1306–14; discussion 14–5
Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 2003 Jan; 61(1): 37–49
Chu FM, Dmochowski R. Pathophysiology of overactive bladder. Am J Med 2006 Mar; 119 (3 Suppl. 1): 3–8
Chess-Williams R, Chapple CR, Yamanishi T, et al. The minor population of M3-receptors mediate contraction of human detrusor muscle in vitro. J Auton Pharmacol 2001 Oct–Dec; 21(5–6): 243–8
Ouslander JG. Management of overactive bladder. N Engl J Med 2004 Feb 19; 350(8): 786–99
Chapple CR, Khullar V, Gabriel Z, et al. The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta-analysis. Eur Urol 2008 Sep; 54(3): 543–62
Abrams P, Andersson KE, Buccafusco JJ, et al. Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br J Pharmacol 2006 Jul; 148(5): 565–78
Ikeda K, Kobayashi S, Suzuki M, et al. M(3) receptor antagonism by the novel antimuscarinic agent solifenacin in the urinary bladder and salivary gland. Naunyn Schmiedebergs Arch Pharmacol 2002 Aug; 366(2): 97–103
Napier C, Gupta P. Darifenacin is selective for the human recombinant M2 receptor subtype [abstract no. 445]. Presented at the 32nd Annual Meeting of the International Continence Society (ICS); 2002 Aug 28–30; Heidelberg
Ney P, Pandita RK, Newgreen DT, et al. Pharmacological characterization of a novel investigational antimuscarinic drug, fesoterodine, in vitro and in vivo. BJU Int 2008 Apr; 101(8): 1036–42
Heading CE. YM-905. Curr Opin CPNS Investig Drugs 2000; 2(3): 321–5
Hegde SS. Muscarinic receptors in the bladder: from basic research to therapeutics. Br J Pharmacol 2006 Feb; 147Suppl. 2: S80–7
Zinner N, Tuttle J, Marks L. Efficacy and tolerability of darifenacin, a muscarinic M3 selective receptor antagonist (M3 SRA), compared with oxybutynin in the treatment of patients with overactive bladder. World J Urol 2005 Sep; 23(4): 248–52
Klausner AP, Steers WD. Antimuscarinics for the treatment of overactive bladder: a review of central nervous system effects. Curr Urol Rep 2007 Nov; 8(6): 441–7
Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006 Jan; 7(1): 41–53
Grant GA, Abbott NJ, Janigro D. Understanding the physiology of the blood-brain barrier: in vitro models. News Physiol Sci 1998 Dec; 13: 287–93
Francis K, van Beek J, Canova C, et al. Innate immunity and brain inflammation: the key role of complement. Expert Rev Mol Med 2003 May; 5(15): 1–19
Madersbacher HG. Confusion about measuring central nervous system effects. Curr Urol Rep 2004 Dec; 5(6): 442–6
Kay GG, Abou-Donia MB, Messer Jr WS, et al. Antimuscarinic drugs for overactive bladder and their potential effects on cognitive function in older patients. J Am Geriatr Soc 2005 Dec; 53(12): 2195–201
Miulli D. Fluid management: the blood-brain barrier and intravenous fluids. In: Siddiqi J, editor. Neurosurgical intensive care. New York (NY): Thieme, 2008: 295–312
de Boer AG, van der Sandt IC, Gaillard PJ. The role of drug transporters at the blood-brain barrier. Annu Rev Pharmacol Toxicol 2003; 43: 629–56
Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease-systematic review and meta-analysis. Neurobiol Aging 2009 Mar; 30(3): 337–52
Diefenbach K, Donath F, Maurer A, et al. Randomised, double-blind study of the effects of oxybutynin, tolter-odine, trospium chloride and placebo on sleep in healthy young volunteers. Clin Drug Investig 2003; 23(6): 395–404
Todorova A, Vonderheid-Guth B, Dimpfel W. Effects of tolterodine, trospium chloride, and oxybutynin on the central nervous system. J Clin Pharmacol 2001 Jun; 41(6): 636–44
Scheife R, Takeda M. Central nervous system safety of anticholinergic drugs for the treatment of overactive bladder in the elderly. Clin Ther 2005 Feb; 27(2): 144–53
Wolters Kluwer Pharma Solutions. Source Lx Managed Care Database. 2010
Katz IR, Sands LP, Bilker W, et al. Identification of medications that cause cognitive impairment in older people: the case of oxybutynin chloride. J Am Geriatr Soc 1998 Jan; 46(1): 8–13
Kay G, Kardiasmenos K, Crook T. Differential effects of the antimuscarinic agents tolterodine tartrate ER and oxybutynin chloride ER on recent memory in older subjects [abstract 87]. Presented at the International Continence Society 36th Annual Meeting; 2006 Nov 27–Dec 1; Christchurch
Sloane PD, Zimmerman S, Brown LC, et al. Inappropriate medication prescribing in residential care/assisted living facilities. J Am Geriatr Soc 2002 Jun; 50(6): 1001–11
Lau DT, Kasper JD, Potter DE, et al. Potentially inappropriate medication prescriptions among elderly nursing home residents: their scope and associated resident and facility characteristics. Health Serv Res 2004 Oct; 39(5): 1257–76
Rigler SK, Jachna CM, Perera S, et al. Patterns of potentially inappropriate medication use across three cohorts of older Medicaid recipients. Ann Pharmacother 2005 Jul–Aug; 39(7–8): 1175–81
Howard M, Dolovich L, Kaczorowski J, et al. Prescribing of potentially inappropriate medications to elderly people. Fam Pract 2004 Jun; 21(3): 244–7
Gray SL, Hedrick SC, Rhinard EE, et al. Potentially inappropriate medication use in community residential care facilities. Ann Pharmacother 2003 Jul–Aug; 37(7–8): 988–93
Roughead EE, Anderson B, Gilbert AL. Potentially inappropriate prescribing among Australian veterans and war widows/widowers. Intern Med J 2007 Jun; 37(6): 402–5
Fialova D, Topinkova E, Gambassi G, et al. Potentially inappropriate medication use among elderly home care patients in Europe. JAMA 2005 Mar 16; 293(11): 1348–58
Barton C, Sklenicka J, Sayegh P, et al. Contraindicated medication use among patients in a memory disorders clinic. Am J Geriatr Pharmacother 2008 Aug; 6(3): 147–52
Raivio MM, Laurila JV, Strandberg TE, et al. Use of inappropriate medications and their prognostic significance among in-hospital and nursing home patients with and without dementia in Finland. Drugs Aging 2006; 23(4): 333–43
Cornford EM, Hyman S. Blood-brain barrier permeability to small and large molecules. Adv Drug Deliv Rev 1999 Apr 5; 36(2–3): 145–63
Liu X, Smith BJ, Chen C, et al. Use of a physiologically based pharmacokinetic model to study the time to reach brain equilibrium: an experimental analysis of the role of blood-brain barrier permeability, plasma protein binding, and brain tissue binding. J Pharmacol Exp Ther 2005 Jun; 313(3): 1254–62
Callegari E, Malhotra B, Bungay PJ, et al. A comprehensive nonclinical evaluation of the CNS penetration potential of antimuscarinic agents for the treatment of overactive bladder. Br J Clin Pharmacol 2011 Mar 11; 72(2): 235–46
Maruyama S, Tsukada H, Yamada S. In vivo analysis of brain muscarinic receptor occupancy after oral oxy-butynin in conscious rhesus monkey by using positron emission tomography (PET) [abstract]. Neurourol Ur-odynam 2007; 26: 703
Pahlman I, d’Argy R, Nilvebrant L. Tissue distribution of tolterodine, a muscarinic receptor antagonist, and transfer into fetus and milk in mice. Arzneimittelforschung 2001 Feb; 51(2): 125–33
Devineni D, Skerjanec A, Woodworth TG. Low central nervous system (CNS) penetration by darifenacin in rats [abstract]. AAPS J 2005; 7: T2277
Staskin D, Kay G, Tannenbaum C, et al. Trospium chloride has no effect on memory testing and is assay undetectable in the central nervous system of older patients with overactive bladder. Int J Clin Pract 2010 Aug; 64(9): 1294–300
Pak RW, Petrou SP, Staskin DR. Trospium chloride: a quaternary amine with unique pharmacologic properties. Curr Urol Rep 2003 Dec; 4(6): 436–40
Sandage B, Lerch G, Larsen G, et al. Trospium chloride does not cross the blood-brain barrier of male Long Evans rats [poster P01.174]. Neurology 2009; 72 (11 Suppl. 3): A50
Enablex (darifenacin extended-release tablets): US prescribing information. Rockaway (NJ): Warner Chilcott (US) LLC, 2010
Steers WD. Darifenacin: pharmacology and clinical usage. Urol Clin North Am 2006 Nov; 33(4): 475–82, viii
Toviaz (fesoterodine fumarate): US prescribing information. New York (NY): Pfizer Inc., 2010
Malhotra B, Wood N, Sachse R, et al. Lipophilicity of 5-hydroxymethyl tolterodine, the active metabolite of fesoterodine [abstract]. Uro Today Int J 2008 Jun; 1 Suppl.: doi:10.3834/uij.1939-4810.2008.06.35
Ditropan XL (oxybutynin chloride) extended release tablets: US prescribing information. Raritan (NJ): Ortho-McNeil-Janssen Pharmaceuticals Inc., 2009
Gamble T, Sand P. Patient perspectives in the management of overactive bladder, focus on transdermal oxybutynin. Patient Prefer Adherence 2008; 2: 349–56
VESIcare® (solifenacin succinate) tablets: US prescribing information. Deerfield (IL): Astellas Pharma US, Inc.; Research Triangle Park (NC): GlaxoSmithKline, 2010
Maniscalco M, Singh-Franco D, Wolowich WR, et al. Solifenacin succinate for the treatment of symptoms of overactive bladder. Clin Ther 2006 Sep; 28(9): 1247–72
Detrol LA (tolterodine tartrate) extended release capsules: US prescribing information. New York (NY): Pfizer Inc., 2009
Abrams P. Evidence for the efficacy and safety of tolterodine in the treatment of overactive bladder. Expert Opin Pharmacother 2001 Oct; 2(10): 1685–701
Sanctura XR (trospium chloride extended release capsules): US prescribing information. Irvine (CA): Allergan, Inc., 2008
Doroshyenko O, Jetter A, Odenthal KP, et al. Clinical pharmacokinetics of trospium chloride. Clin Pharmaco-kinet 2005; 44(7): 701–20
Michel MC, Hegde SS. Treatment of the overactive bladder syndrome with muscarinic receptor antagonists: a matter of metabolites? Naunyn Schmiedebergs Arch Pharmacol 2006 Nov; 374(2): 79–85
Maruyama S, Tsukada H, Nishiyama S, et al. In vivo quantitative autoradiographic analysis of brain muscarinic receptor occupancy by antimuscarinic agents for overactive bladder treatment. J Pharmacol Exp Ther 2008 Jun; 325(3): 774–81
Gulsun M, Pinar M, Sabanci U. Psychotic disorder induced by oxybutynin: presentation of two cases. Clin Drug Investig 2006; 26(10): 603–6
Nye AM, Clinard VB, Barnes CL. Medication non-adherence secondary to drug-induced memory loss. Consult Pharm 2010 Feb; 25(2): 117–21
Womack KB, Heilman KM. Tolterodine and memory: dry but forgetful. Arch Neurol 2003 May; 60(5): 771–3
Tsao JW, Heilman KM. Transient memory impairment and hallucinations associated with tolterodine use. N Engl J Med 2003 Dec 4; 349(23): 2274–5
Salvatore S, Serati M, Cardozo L, et al. Cognitive dysfunction with tolterodine use [abstract]. Am J Obstet Gynecol 2007 Aug; 197(2): e8
Giramonti KM, Kogan BA, Halpern LF. The effects of anticholinergic drugs on attention span and short-term memory skills in children. Neurourol Urodyn 2008; 27(4): 315–8
Kay GG, Wesnes KA. Pharmacodynamic effects of darifenacin, a muscarinic M selective receptor antagonist for the treatment of overactive bladder, in healthy volunteers. BJU Int 2005 Nov; 96(7): 1055–62
Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res 2001 Jul; 11(7): 1156–66
Begley DJ. ABC transporters and the blood-brain barrier. Curr Pharm Des 2004; 10(12): 1295–312
Edwards G. Ivermectin: does P-glycoprotein play a role in neurotoxicity? Filaria J 2003; 2Suppl. 1: S1–8
Geyer J, Gavrilova O, Petzinger E. The role of P-glycoprotein in limiting brain penetration of the peripherally acting anticholinergic overactive bladder drug trospium chloride. Drug Metab Dispos 2009 Jul; 37(7): 1371–4
Geyer J, Gavrilova O, Schwantes U. Differences in the brain penetration of the anticholinergic drugs trospium chloride and oxybutynin. UroToday Int J 2010; 3(1): doi:10.3834/uij.1944-5784.2010.02.12
Sandage B, Sabounjian L, Shipley J, et al. Predictive power of an in vitro system to assess drug interactions of an antimuscarinic medication: a comparison of in vitro and in vivo drug-drug interaction studies of trospium chloride with digoxin. J Clin Pharmacol 2006 Jul; 46(7): 776–84
Obradovic T, Dobson GG, Shingaki T, et al. Assessment of the first and second generation antihistamines brain penetration and role of P-glycoprotein. Pharm Res 2007 Feb; 24(2): 318–27
Kimchi-Sarfaty C, Oh JM, Kim IW, et al. A ‘silent’ polymorphism in the MDR1 gene changes substrate specificity. Science 2007 Jan 26; 315(5811): 525–8
Hoffmeyer S, Burk O, von Richter O, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci U S A 2000 Mar 28; 97(7): 3473–8
Chancellor MB, de Miguel F. Treatment of overactive bladder: selective use of anticholinergic agents with low drug-drug interaction potential. Geriatrics 2007 May; 62(5): 15–24
Abdel-Rahman A, Shetty AK, Abou-Donia MB. Disruption of the blood-brain barrier and neuronal cell death in cingulate cortex, dentate gyrus, thalamus, and hypothalamus in a rat model of Gulf-War syndrome. Neurobiol Dis 2002 Aug; 10(3): 306–26
Esposito P, Gheorghe D, Kandere K, et al. Acute stress increases permeability of the blood-brain-barrier through activation of brain mast cells. Brain Res 2001 Jan 5; 888(1): 117–27
Zipser BD, Johanson CE, Gonzalez L, et al. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol Aging 2007 Jul; 28(7): 977–86
Bronge L, Wahlund LO. White matter lesions in dementia: an MRI study on blood-brain barrier dysfunction. Dement Geriatr Cogn Disord 2000 Sep–Oct; 11(5): 263–7
Starr JM, Wardlaw J, Ferguson K, et al. Increased blood-brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J Neurol Neurosurg Psychiatry 2003 Jan; 74(1): 70–6
Minagar A, Alexander JS. Blood-brain barrier disruption in multiple sclerosis. Mult Scler 2003 Dec; 9(6): 540–9
Bennett J, Basivireddy J, Kollar A, et al. Blood-brain barrier disruption and enhanced vascular permeability in the multiple sclerosis model EAE. J Neuroimmunol 2010 Dec 15; 229(1–2): 180–91
Borges N, Shi F, Azevedo I, et al. Changes in brain microvessel endothelial cell monolayer permeability induced by adrenergic drugs. Eur J Pharmacol 1994 Oct 14; 269(2): 243–8
Bartus RT, Elliott PJ, Dean RL, et al. Controlled modulation of BBB permeability using the bradykinin agonist, RMP-7. Exp Neurol 1996 Nov; 142(1): 14–28
Black KL, Yin D, Ong JM, et al. PDE5 inhibitors enhance tumor permeability and efficacy of chemotherapy in a rat brain tumor model. Brain Res 2008 Sep 16; 1230: 290–302
Robinson PJ. Measurement of blood-brain barrier permeability. Clin Exp Pharmacol Physiol 1990 Dec; 17(12): 829–40
Oka T, Nakano K, Kirimoto T, et al. Effects of anti-muscarinic drugs on both urinary frequency and cognitive impairment in conscious, nonrestrained rats. Jpn J Pharmacol 2001 Sep; 87(1): 27–33
Sugiyama T, Park YC, Kurita T. Oxybutynin disrupts learning and memory in the rat passive avoidance response. Urol Res 1999; 27: 393–5
Suzuki M, Noguchi Y, Okutsu H, et al. Effect of anti-muscarinic drugs used for overactive bladder on learning in a rat passive avoidance response test. Eur J Pharmacol 2007 Feb 28; 557(2–3): 154–8
Cappon GD, Bush B, Newgreen D, et al. Tolterodine does not affect memory assessed by passive-avoidance response test in mice. Eur J Pharmacol 2008 Jan 28; 579(1–3): 225–8
McLeod-Flynn L, Reid L. Center for Drug Evaluation and Research pharmacology review: trospium chloride [application number 21-595]. Silver Spring (MD): Department of Health and Human Service, Public Health Service, Food and Drug Administration, 2004
Feng B, Mills JB, Davidson RE, et al. In vitro P-glycoprotein assays to predict the in vivo interactions of P-glycoprotein with drugs in the central nervous system. Drug Metab Dispos 2008 Feb; 36(2): 268–75
Turvey CL, Schultz S, Arndt S, et al. Memory complaint in a community sample aged 70 and older. J Am Geriatr Soc 2000 Nov; 48(11): 1435–41
Demers M, Rouleau I, Scherzer P, et al. Impact of the cognitive status on the memory complaints in MS patients. Can J Neurol Sci 2011 Sep; 38(5): 728–33
Slavin MJ, Brodaty H, Kochan NA, et al. Prevalence and predictors of ‘subjective cognitive complaints’ in the Sydney Memory and Ageing Study. Am J Geriatr Psychiatry 2010 Aug; 18(8): 701–10
Wesnes KA, Edgar C, Tretter RN, et al. Exploratory pilot study assessing the risk of cognitive impairment or sedation in the elderly following single doses of solifenacin 10mg. Expert Opin Drug Saf 2009 Nov; 8(6): 615–26
Kay G, Crook T, Rekeda L, et al. Differential effects of the antimuscarinic agents darifenacin and oxybutynin ER on memory in older subjects. Eur Urol 2006 Aug; 50(2): 317–26
Lipton RB, Kolodner K, Wesnes K. Assessment of cognitive function of the elderly population: effects of darifenacin. J Urol 2005 Feb; 173(2): 493–8
Herberg K, Fusgen I. Effect of trospium chloride, oxybutynin-HCL and propiverin-HCL on traffic related performance with respect to safety. Geriat Forsch 1997; 7(2): 77–83
Herberg K. Everyday life safety and road safety after treatment with drugs against incontinence: Recent studies on the safety potential of urologic anticholinergics. Die Medizinische Welt 1999; 50(4–5): 217–22
Diefenbach K, Arold G, Wollny A, et al. Effects on sleep of anticholinergics used for overactive bladder treatment in healthy volunteers aged > or =50 years. BJU Int 2005 Feb; 95(3): 346–9
Staskin DR, Harnett MD. Effect of trospium chloride on somnolence and sleepiness in patients with overactive bladder. Curr Urol Rep 2004 Dec; 5(6): 423–6
Pietzko A, Dimpfel W, Schwantes U, et al. Influences of trospium chloride and oxybutynin on quantitative EEG in healthy volunteers. Eur J Clin Pharmacol 1994; 47(4): 337–43
Perry EK, Kilford L, Lees AJ, et al. Increased Alzheimer pathology in Parkinson’s disease related to antimuscarinic drugs. Ann Neurol 2003 Aug; 54(2): 235–8
Acknowledgements
Mary Hines, Ray Hill and Alan J. Klopp, PhD, of inScience Communications, provided editorial support, which was funded by Allergan, Inc.
Dr Michael B. Chancellor is a consultant and investigator for and has received honoraria from Allergan, Inc., Astellas Pharma US, Inc., Pfizer and Ono Pharmaceuticals and has received grants from Pfizer and Medtronic. Dr David R. Staskin is a consultant for Allergan, Inc., Astellas Pharma US, Inc., GlaxoSmithKline and Pfizer and a speaker for and/or has received honoraria from Allergan, Inc., Astellas Pharma US, Inc., GlaxoSmithKline, Pfizer and Watson Pharmaceuticals. Dr Gary G. Kay is a consultant and speaker for and has received grants and honoraria from Allergan, Inc., Novartis, Pfizer and Watson Pharmaceuticals. Dr Bobby W. Sandage, Jr, was formerly an employee of Covidien Pharmaceuticals and Indevus. Dr Michael G. Oefelein was an employee of Allergan, Inc. at the time this manuscript was written. Dr Jack W. Tsao has no potential conflicts of interest to declare. In the US, Allergan, Inc. manufactures and distributes trospium chloride (Sanctura®), Astellas Pharma US, Inc. manufactures and distributes solifenacin (VESIcare®), Novartis manufactures and distributes darifenacin (Enablex®), Pfizer Inc. distributes fesoterodine (Toviaz®) and tolterodine (Detrol®), and Ortho-McNeil-Janssen Pharmaceuticals Inc. manufactures and distributes oxybutynin chloride (Ditropan®).
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the United States Department of the Navy or the Department of Defense.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chancellor, M.B., Staskin, D.R., Kay, G.G. et al. Blood-Brain Barrier Permeation and Efflux Exclusion of Anticholinergics Used in the Treatment of Overactive Bladder. Drugs Aging 29, 259–273 (2012). https://doi.org/10.2165/11597530-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11597530-000000000-00000