Abstract
Inhaled nitric oxide (iNO) has emerged as a promising therapeutic agent in the treatment of persistent pulmonary hypertension of the newborn. Several theories exist regarding causes of both response and nonresponse to iNO. Clinical trials differentiate disease entities (primary vs secondary persistent pulmonary hypertension associated with meconium aspiration syndrome, pneumonia or congenital diaphragmatic hernia) and their specific response rates. iNO combined with high-frequency ventilation appears to be superior to inhalation of nitric oxide (NO) during conventional ventilation. Little is known regarding the role of the degree of lung expansion and its modification — no matter what mode of ventilation is applied. Gestational age plays an important role in relation to the potential adverse effects of NO. Of particular concern in the premature neonate is the effect of NO on bleeding time and the inhibition of platelet aggregation. Those potentially hazardous effects need to be carefully weighed against early intervention with iNO at a comparably low oxygenation index in order to prevent the vicious cycle of hypoxaemia and subsequent increased right-to-left shunting. Further studies are required to determine the optimal timing, mode of delivery and mode of ventilation used with iNO therapy in order to optimise the response of premature and term neonates.
Similar content being viewed by others
Inhaled nitric oxide (iNO) for the treatment of persistent pulmonary hypertension (PPHN) of the newborn has been in use since the beginning of the 1990s, when first reports of successful applications in neonates were published.[1,2] Since then numerous cases of predominantly beneficial applications of iNO in neonates with pulmonary hypertension secondary to various underlying diseases have been reported. Several randomised, prospective studies investigating the effects of iNO in the treatment of pulmonary hypertension in term neonates show an improvement in oxygenation.[3–6] Although less data are available concerning the situation in premature neonates, response or nonresponse to iNO occurs in a comparable frequency depending on the underlying disease.[7–9] Particular concern has been raised by reports concerning the potential of an increased rate of intraventricular haemorrhage (IVH) in preterm neonates following iNO therapy.[8] iNO leads to a prolonged bleeding time and an inhibition of platelet aggregation in adult volunteers[10] and in patients with acute respiratory distress syndrome (ARDS).[11] This has only recently been shown to be also the case in preterm and term neonates.[12,13] As a result, considerable caution has replaced the initial enthusiasm towards the unrestricted use of iNO, particularly in preterm neonates. This review evaluates the available new information from clinical trials and laboratory investigations.
1. Inhaled Nitric Oxide (iNO) in Term Neonates
First reports regarding the successful application of iNO in term neonates came from Roberts et al.[1] and Kinsella et al.[2] in 1992. The former used an iNO concentration of 80ppm and found a rapid increase in oxygen saturation and oxygen tension, the latter applied lower iNO dosages (10 to 20ppm), which also led to rapid improvement in oxygenation in all neonates without reduction of systemic blood pressure. The common message of both reports was reversal of extrapulmonary right-to-left shunting and improvement in oxygenation with an unaffected systemic blood pressure due to selective pulmonary vasodilation.
Indications for the use of iNO in term neonates extended subsequently to neonates with pulmonary hypertensive episodes following surgery,[14] cardiac surgery[15,16] and neonates with congenital hypoplasia of the lungs secondary to oligohydramnios.[17]
In classical PPHN the success of iNO therapy was defined by the number of neonates avoiding treatment with extracorporeal membrane oxygenation (ECMO). Hoffman et al.[18] reported a significant decrease in ECMO utilisation in their patients. In 50 neonates of ≥35 weeks of gestation with PPHN the authors compared 2 groups of neonates: those treated before the availability of iNO and those treated after. They found a 67% reduction in the number of neonates treated with ECMO. However, these results should be treated with caution because of the use of a historical control group. Attributing the significant differences exclusively to treatment with iNO would not take into account recent advances in obstetrics related to the management and timing of optimal delivery in an at risk population.
Day et al.[19] included 50 newborns with respiratory failure and pulmonary hypertension in their prospective trial. Neonates with an oxygenation index (OI) between 25 and 40 were randomised to receive conventional therapy with or without 20ppm nitric oxide (NO). All neonates with an OI above 40 were treated with iNO. High-frequency jet ventilation was used in some of the patients with pulmonary interstitial emphysema, and in these neonates arterial oxygen tension (PaO2) levels were significantly lower. The authors found an improvement in oxygenation in the majority of neonates treated with iNO. Oxygenation was less likely to improve in neonates with lung hypoplasia or those with radiographic evidence of severe diffuse parenchymal lung disease.
Barefield et al.[20] randomised 17 term and near term neonates with hypoxaemic respiratory failure to receive either up to 80ppm iNO or conventional therapy. Concomitant high-frequency ventilation (HFV) and surfactant therapy were not allowed, in cases of treatment failure crossover of the control neonates to receive iNO was permitted. Following treatment failure in iNO-treated neonates a trial of HFV was performed before proceeding to ECMO. No differences between the groups could be shown since ultimately all neonates required ECMO.
The Neonatal Inhaled Nitric Oxide Study Group (NINOS) reported their randomised, multicentre, controlled trial in early 1997.[5] This study enrolled 235 neonates with a gestational age of ≥34 weeks, who required mechanical ventilation and had an OI of 25 or above. Two different concentrations of NO (20ppm and 80ppm) were applied, the control neonates received 100% oxygen. Mortality was comparable between controls and iNO-treated neonates (17 vs 14%), but significantly fewer neonates in the NO group required ECMO (39 vs 54%).
Roberts et al.[3] included 58 full term neonates with severe hypoxaemia and PPHN in a randomised controlled trial. Patients were randomly assigned to breathe either a control gas or NO 80ppm. If iNO therapy was considered successful, treatment was continued at lower concentrations. Otherwise, alternative treatment options including ECMO were used. The authors found an improvement in oxygenation in the majority of neonates treated with iNO. Mortality was similar in the two groups. ECMO was required in 71% of the control group, but in only 40% of the treatment group.
In a prospective, randomised, single centre study by Wessel et al.,[6] 49 mechanically ventilated neonates with clinical and echocardiographic evidence of PPHN were randomised to treatment with or without iNO. Primary outcome variables included oxygenation, mortality and use of ECMO. The majority of neonates had diagnoses of either meconium aspiration syndrome (MAS) or primary PPHN, diagnoses were equally distributed between treatment groups. PaO2, oxygen saturation, and OI improved in the iNO group, the median percent change in OI (−31%) in the iNO group was significantly different from baseline and from the control group. Neither mortality nor the use of ECMO was affected by NO treatment. The authors speculated that the use of specific lung expansion strategies with the use of iNO might lead to a reduced use of ECMO in neonates with PPHN.
The latter speculation might well have its origin in the preliminary results of other, simultaneously ongoing studies of iNO in neonates, which permitted the use of either high-frequency oscillatory ventilation (HFOV) or conventional strategies aimed at optimisation of lung volume. At the stage of publication of the first studies of iNO in the newborn, cumulative evidence began to appear which indicated a superior effect of a combination of iNO and HFOV. What appeared purely coincidental on the initial description, was soon justified on a pathophysiological basis: the more alveoli were open, the more NO found its way to the vascular smooth muscle cell. This in turn led to a more pronounced increase in pulmonary blood flow and subsequently to an improvement in oxygenation. Although the majority of prospective randomised controlled trials (RCT) of iNO in neonates did not permit the use of HFOV during iNO therapy, 2 studies included HFOV in their study design.
One of these studies by Kinsella et al.[21] included 205 neonates with PPHN in a randomised, multicentre trial of HFOV versus iNO during conventional ventilation. Crossover to the alternative treatment was permitted in cases of nonresponse. Treatment failure after crossover led to a combined treatment with HFOV and iNO. Patients were stratified by predominant disease category: respiratory distress syndrome (RDS), MAS, idiopathic PPHN and congenital diaphragmatic hernia (CDH). Initial response rate to the assigned treatment modality was comparable between the HFOV and the iNO group. Following crossover to the alternate treatment, response rates again were not significantly different. One third of the 125 patients in whom both treatment modalities failed, responded favourably to the combined therapy with iNO and HFOV. Analysed by disease category, response rates for HFOV plus iNO in patients with RDS (47%) and with MAS (38%) were significantly better than for HFOV alone (32 and 16%) or iNO with conventional ventilation (32 and 22%).
Davidson et al.[22] reported the results of a randomised, placebo-controlled, double-blind, dose-response, multicentre study which was terminated at the inclusion of 155 patients because of slow patient recruitment. Term neonates (≥37 weeks) with birth weights of ≥2500g and requiring mechanical ventilation with a fraction of inspired oxygen (FiO2) of 1.0 within the first 72 hours of life were randomised. Patients were excluded if they had received surfactant therapy or had been treated with HFV within 6 hours of starting study gas treatment. Significant increases in PaO2 as well as reduction of the OI were recorded in the iNO group. The primary end-point was a PPHN Major Sequelae Index (MSI), including the incidence of death, use of ECMO and neurological injury. MSI was not different between the two groups. Although a trend towards a decreased use of ECMO in the iNO group was noted, this effect did not reach statistical significance.
For further details of the largest published trials in term neonates see table I.
In summary, the majority of term neonates with respiratory failure do respond favourably to iNO (with the exception of neonates with CDH). In their meta-analysis of randomised trials of iNO, Finer and Barrington[23,24] found a significant reduction in the incidence of death or need for ECMO. Since the incidence of death was comparable in the groups, the effect of reduced need for ECMO was attributable to iNO treatment. Well known adverse effects of NO inhalation should be continuously monitored for, but appear to lose their menace in the light of a trend towards the clinical use of lower doses.
2. Response Rate Related to Underlying Disorder
In a series of 25 near term neonates with severe PPHN (OI >25), Goldman et al.[25] reported 4 different patterns of response to iNO therapy: nonresponse, initial response but failure to sustain this effect beyond 36 hours, sustained response with subsequent successful weaning from iNO, and response at high doses of iNO with sustained dependence on iNO. The latter group comprised neonates with congenital hypoplasia and dysplasia of the lung, and mortality in these neonates was 100%. Sustained responders and early failures covered the whole spectrum of PPHN-associated diagnoses such as MAS, RDS, CDH, sepsis and idiopathic PPHN without parenchymal lung disease. There was no obvious predictive value of the underlying lung disease determining a sustained response to iNO. The 2 neonates in the nonresponse group both had MAS, one of which had received surfactant and a trial of HFOV. Unfortunately, important details are missing with regard to the simultaneous treatment with surfactant, HFOV and iNO in order to understand why the combined treatment did not yield the desired effect. A positive response to iNO would appear more likely after these lung expansion modalities (surfactant, HFOV). This has been shown in an animal model of meconium aspiration in pigs, which were treated with surfactant prior to iNO therapy.[26] Kinsella et al.[21] reported a superior effect of iNO in combination with HFOV. This effect was most striking in a subgroup of neonates with MAS and RDS. There was no synergistic effect in a group with idiopathic PPHN, lung hypoplasia or CDH. The latter finding corresponds with the results of the NINOS.[4] Although improvements in oxygenation occurred in some neonates, the authors found no reduction in the need for ECMO or death in the iNO treatment group.
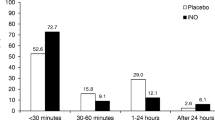
In their study, Mercier et al.[27] described a disease-related response to iNO. Both premature and term neonates with hypoxaemic respiratory failure were enrolled. Within 30 minutes of iNO therapy OI improved significantly in preterm neonates, neonates with idiopathic PPHN or ARDS, and sepsis. Less pronounced was the response in neonates with MAS, and much less in those with CDH. Although the authors permitted the use of conventional ventilation or HFV, they did not stratify their results for the mode of ventilation.
Neonates with alveolar capillary dysplasia and subsequent PPHN were found to initially respond favourably to iNO.[28] Unfortunately, this effect was not sustained, all neonates in this series ultimately required iNO in concentrations of 80ppm or above in order to maintain adequate oxygenation. Approximately 80% of those neonates required ECMO treatment and eventually they all died.
Adler and Clark[29] compared the dose-response relationship applying various concentrations of iNO between premature and term neonates. They found similar response curves for the 2 groups in the range between 10 and 40ppm. Beyond 40ppm no additional benefit in oxygenation parameters was demonstrable, nonresponse was associated with a poor prognosis independent of gestational age.
Summarising the effect of the underlying pulmonary disorder on the response rate during iNO treatment requires taking a closer look at disease related differences in alveolar ventilation. Various degrees thereof might represent the most decisive parameter for response or nonresponse to iNO. Pure hypoxia associated PPHN without additional parenchymal lung disease usually presents with unrestricted alveolar ventilation and therefore a high rate of clinical response to iNO. Whereas in neonatal pneumonia, the limited aeration of the lung makes it easy to imagine the difficulty for NO to diffuse to a substantial number of alveoli in order to positively affect the patient’s oxygenation. Hypoplasia of the lung (including CDH) represents another special scenario: even if the majority of existing alveoli were recruited, this might not be sufficient to ensure oxygenation of the newborn. Inhomogeneity of lung expansion due to the use of inadequate lung recruitment strategies might lead to a higher than usual degree of ventilation-perfusion mismatching. The reversal of the associated intrapulmonary shunting contributes less to an improvement in oxygenation than the reversal of extrapulmonary shunting via the ductus or the foramen ovale. Therefore a limited response to iNO in pulmonary hypoplasia of whatever cause would be expected.
3. iNO in Preterm Neonates
PPHN of the newborn has, for a long time, been regarded as a disease exclusively present in term or post-term neonates. In the mid 1980s the first reports of premature neonates with PPHN, as evidenced by right-to-left shunting via ductus arteriosus and/or foramen ovale, appeared.[30,31] Since then there have been various reports of PPHN in preterm neonates associated with RDS, but also with all other disease states well known in term neonates.
Surfactant replacement therapy represents the causative treatment for preterm neonates with surfactant deficient lungs. Surfactant therapy also leads to alveolar recruitment which facilitates diffusion of iNO to the vascular endothelium adjacent to the alveolus. This effect has been shown by Gommers et al.[32] in a lung lavage model in rabbits. The authors found a more pronounced effect of iNO on oxygenation if there was pre-recruitment of alveoli by previous delivery of surfactant rather than by increasing the level of positive end-expiratory pressure (PEEP) up to 10cm H2O.
It has to be kept in mind that neonates with a gestational age in the upper range of prematurity (i.e. 34 to 36 weeks) were included in some of the large studies discussed in sections 1 and 2.[5,6,19–21] This is important for 2 reasons: first, these neonates have the option according to their bodyweight and gestational age to proceed to ECMO if required. Secondly, the absolute risk of experiencing the classical IVH is fairly small at this near term gestational age.
Contrary to this, Peliowski et al.[33] reported their experience with iNO therapy in 8 premature neonates between 24 and 31 weeks of gestation, whose mothers had prolonged rupture of membranes and oligohydramnios. All neonates received a FiO2 of 1.0, a mean airway pressure (MAP) of ≥14cm H2O and had an OI of ≥19 on either conventional or HFV. Significant reductions of OI and MAPs could be noted during NO inhalation. Three out of 8 neonates died, 2 of which had experienced grade 4 IVHs. Because of acute deterioration of the neonates’ condition, cranial ultrasound examinations were not available for all of them.
In their study, Subhedar et al.[7] included 42 neonates with a gestational age of less than 32 weeks, who were recruited at an age of 96 hours if they were deemed to be at high risk of developing bronchopulmonary dysplasia (BPD). Neonates were randomised to receive either iNO or dexamethasone, or both or neither treatment modality. Study end-points were chronic lung disease (CLD) or death. Cranial ultrasound was performed in all neonates before randomisation and subsequently on a regular basis. IVHs less than grade 4 were not regarded as a contraindication to treatment with iNO. The authors found no progression of a pre-existing IVH in the iNO group, but in the control group. There was no effect on the incidence of CLD or death with either treatment. The authors concluded that treatment with iNO in a high risk group of preterm neonates was not associated with a significant increase in adverse events. This study by Subhedar and colleagues[7] is unique because it compares an already more or less established therapy (dexamethasone for the prevention or treatment of BPD) with a therapy aimed at reducing pulmonary artery pressure and improving ventilation-perfusion mismatching (iNO). Increased pulmonary vascular resistance (PVR) and V/Q-mismatching are typical features of severe BPD. The major restriction of the study by Subhedar et al. is the small number of neonates randomised, which is aggravated by the fact that the authors have chosen to compare 4 different treatment modalities. With regard to the progression of preexisting IVHs, this study does not contribute to answer either the question of safety of iNO in preterm neonates or that of a potential IVH-protective effect of NO. The latter effect could be deducted from the avoidance of V/Q-mismatching, frequent changes in pulmonary artery pressures and systemic blood pressure, and subsequent hypoxaemia, which in turn would lead to further pulmonary vasoconstriction. In order to clarify the role of NO-induced inhibition of platelet aggregation and its effect on pre-existing haemorrhages, clearly large numbers of neonates are needed. Since the numbers of potential candidates for enrolment in such a study are limited, a substantial international collaboratory effort would be a prerequisite for the initiation of such a multicentre trial.
Van Meurs et al.[8] included 11 neonates in a small, sequential study of iNO at 4 different concentrations and placebo. Ten of the 11 neonates had a greater than 25% increase in PaO2/PAO2. Although none of the neonates had evidence of IVH at their initial cerebral ultrasound examination, 3 of them had proof of IVH after NO treatment and 4 additional neonates developed IVH during the further course of hospitalisation.
Skimming et al.[9] compared the effect of 5ppm versus 20ppm iNO in 23 preterm neonates with a diagnosis of RDS. They found an improvement in oxygenation parameters with both concentrations of iNO. Unfortunately no information was given regarding cranial ultrasound examinations either before or after iNO treatment.
Banks et al.[34] reported their experience with iNO therapy in neonates with severe ventilator dependent BPD. Patients older than 4 weeks were enrolled if they required a MAP ≥10 cmH2O and an FiO2 of ≥0.45. The authors showed that 7 out of 11 neonates responded favourably (as defined by a reduction of FiO2 of >15%), mortality was 36%, which was clearly lower than the expected mortality of ≥75%.
Cheung et al.[35] reported on the neurodevelopmental outcome of very low birth weight neonates treated with iNO. Although significant improvements in arterial oxygenation of these patients occurred, the mortality rate was substantial (58%). The survivors were examined at 13 to 40 months of adjusted age. Of these remaining neonates 50% were disabled, 20% were developmentally retarded and 30% had a normal development. Mortality as well as morbidity in this particular group of patients is generally regarded as high and would be expected to represent the severity of the disease rather than an effect of iNO treatment. The timing of episodes of pulmonary hypertension and the most frequent timing for the occurrence of IVHs coincide in preterm neonates. This leads to an assumed causal relationship of iNO treatment and IVH, where the course of the underlying disease leading to PPHN might pose a sufficiently high risk for the occurrence of IVH. This view is supported by the study of Subhedar et al.,[7] who found no progression in any preterm neonate treated with iNO and pre-existing IVH.
Kinsella et al.[36] reported the results of a multicentre study, which was performed in a double-blinded and randomised manner. 80 neonates with a gestational age of ≤34 weeks with severe hypoxaemic respiratory failure were randomised to receive iNO at 5ppm or not. The primary outcome was survival to discharge, secondary outcome measures included rate and severity of intracranial haemorrhage, pulmonary haemorrhage, duration of ventilation and CLD. Apart from gestational age, enrolment criteria were: postnatal age of less than 7 days, severe hypoxaemia (arterial/alveolar oxygen ratio <0.10) despite mechanical ventilation and surfactant replacement when indicated. Ventilatory support was provided by time-cycled, pressure-limited ventilators or with high-frequency devices. During HFV, a high-volume strategy was used by convention in all centres. The authors found an improvement in oxygenation after 60 minutes in the iNO group (p = 0.03). Survival was not different between the groups (52% in the iNO group vs 47% in controls). Adverse outcomes were also comparable between groups (intracranial haemorrhage grade 2 to 4: 28% iNO vs 33% control; pulmonary haemorrhage: 13 vs 9%; CLD: 60 vs 80%).
In the study of the Franco-Belgium Collaborative NO Trial Group,[37] 204 neonates were randomised to receive iNO at 10ppm or conventional ventilation without iNO. Entry criteria were stratified for gestational age: preterm neonates <33 weeks required an OI between 12.5 and 30, neonates ≥33 weeks required an OI between 15 and 40 in order to qualify for recruitment. The primary end-point was the OI at 2 hours, secondary end-points included the number of days on mechanical ventilation and oxygen therapy, the length of stay in the neonatal intensive care unit (NICU) and hospital, the incidence of CLD requiring therapy with either β2 agonists or corticosteroids, and the occurrence of severe intracranial haemorrhage, cystic leucomalacia and death. Preterm neonates were given exogenous surfactant, a high-volume ventilatory strategy was applied with either conventional ventilation or HFV. Neonates with a gestational age ≥33 weeks had a significant reduction in the OI, duration of mechanical ventilation and duration of NICU stay. This was not the case in the group of neonates <33 weeks where iNO was not significantly beneficial. Secondary outcome measures also showed no differences between both groups (alive without neurological complications: 38% controls vs 40% iNO; intracranial haemorrhage and cystic leucomalacia: 27% controls vs 32% iNO; deaths occurring in NICU: 35% controls vs 27% iNO).
In summary, the predominant lung expansion strategy applied in premature neonates is the instillation of exogenous surfactant. This is evident from basically all studies related to premature neonates, including a meta-analysis by Barrington and Finer.[38] There is a trend towards a more frequent use of HFOV in preterm neonates, which might be related to the fact that ECMO is not available for the majority of these patients. The main limitation to the widespread use of iNO is its potential effect on the coagulation system. This ambiguous situation clearly warrants further investigation.
For further details of selected trials in preterm neonates see table II.
4. Adverse Effects
Adverse effects of iNO include haemorrhagic diathesis due to inhibition of platelet aggregation,[10–13] nitrogen dioxide (NO2) formation and subsequent potential pulmonary toxicity,[39] peroxynitrite formation and its effect on DNA,[40] inhibition of bacterial growth,[41] and methaemoglobinaemia.[3,5,6,22] Of particular interest in premature neonates at risk of IVH is the potential effect of iNO on platelet aggregation. Whereas in initial studies cerebral ultrasound scans were not performed on a regular basis,[33] other investigators have done so without reporting either an increased incidence of IVH or a progression of pre-existing haemorrhages.[7] Van Meurs et al.[8] reported an unusually high incidence of IVH in their series of 11 neonates. The randomisation of their patients was performed at a postnatal age of 24 to 72 hours at which stage all neonates had normal sonographic findings of their cerebral anatomy. The fact that 3 of the neonates had proof of IVH following a 2-hour exposure to various levels of iNO does not necessarily represent a causal relationship. Since this time window corresponds with the classical timing of IVH in premature neonates, much larger numbers of neonates would have to be compared with a non-iNO—treated control group in order to clarify the role of NO in the pathophysiology of IVH.
Cheung et al.[12,35] showed an inhibition of platelet aggregation in premature and term neonates treated with iNO, which was accompanied by a raised intraplatelet level of cyclic guanosine 5′-monophosphate. This effect on platelet aggregation was sustained for up to 24 hours, 2 of the premature neonates in this group developed severe IVH. According to their data, iNO might at least represent a contributory factor in the pathogenesis or determination of the size of IVH.
A study performed by George et al.[13] showed a nearly 2-fold increase in bleeding time in neonates treated with iNO at 40ppm for 30 minutes. No significant differences in the platelet aggregation responses to various agonists during and following iNO exposure were identified. The most important difference between patients in this study and those of Cheung et al.[12] is gestational age: whereas George et al.[13] included only one neonate with a gestational age of less than 35 weeks, the majority of patients in Cheung’s study were premature low birth weight neonates. This difference might explain the inconclusive results of these 2 studies.
Christou et al.[42] found no inhibition of the in vitro activation of platelets derived from newborns with a gestational age ≥34 weeks. Although rapid inactivation of iNO by binding to haemoglobin takes place, platelets are directly exposed to iNO and their function might be affected by it. Which, by the way, would establish a sophisticated mediator system for effects of modified peripheral blood cells at the site of distant organs.
The Franco-Belgium Collaborative NO Trial Group[37] provided the largest study of iNO in preterm neonates and report results of a multicentre trial conducted in France and Belgium. In this randomised clinical trial of 83 neonates with a gestational age of 32 weeks or less, there was a trend towards a decreased number of intracranial haemorrhages, leukomalacia and death in the iNO treatment group. These data represent the largest series of neonates enrolled at a comparable gestational age until now and this investigation appears to supply sufficient evidence to assume an acceptable level of safety among premature neonates treated with iNO.
Hallman et al.[40] prospectively analysed airway specimens from 24 newborn neonates. They found that iNO exposure at ≤20ppm did not affect the concentrations of the lipid peroxidation product, the surface activity or cytokines (interleukin-1, granulocyte-macrophage colony-stimulating factor, interleukin-1 receptor antagonist). The investigators also looked for toxic effects of iNO, which is known to form peroxynitrite in the presence of superoxide.[43] Peroxynitrite reacts with the phenolic residues of tyrosines to form nitrotyrosine. In Hallman’s study,[40] nitrotyrosine could only be detected after 10 days of life in 2 neonates requiring prolonged ventilation without iNO. They suggested that the source of nitrotyrosine in these 2 patients must be endogenous NO production.
In summary, the clinical trials conducted so far are not indicative of an increased incidence of haemorrhagic complications in term neonates. The numbers of premature neonates included in randomised trials are too small to draw substantial conclusions from the results. On the background of an increase in bleeding time in combination with a pre-existing IVH, the severity of such a haemorrhage could be much greater than without interference of this iNO-related adverse effect. Again this represents an argument for the necessity of a randomised controlled trial of iNO in preterm neonates. How difficult it may be to design and conduct such a study has been shown only recently, when a European multicentre study was stopped because of slow recruitment of patients. A future study design clearly has to handle main determining factors related to the efficacy of iNO (i.e. mode of ventilation) in a liberal way in order to include the maximum number of patients.
For further details of the most common adverse effects of NO see table III.
5. Response Rate Related to Mode of Ventilation
Although mechanisms leading to response or nonresponse to iNO are still incompletely understood, insufficient lung inflation with persistence of alveolar collapse is the most common cause of treatment failure.[55] In order to prevent insufficient lung expansion, a variety of alveolar recruitment strategies have been used. These strategies consist of either modifications of the level of PEEP during conventional mechanical ventilation, or of an increase of MAP during HFV.[21,56,57] In a multicentre trial, Kinsella et al.[21] randomised 205 neonates with PPHN to treatment with iNO and conventional ventilation or to HFOV without iNO. Treatment failure resulted in crossover to the alternative therapeutic modality. Treatment failure following crossover led to a combined treatment with HFOV and iNO. Altogether they found 43 neonates who were nonresponders to either treatment alone, but responded favourably to the combined application of iNO and HFOV.
An animal model using dogs with oleic acid-induced lung injury showed that a recruitment of gas exchange units with continous positive airway pressure (CPAP) during conventional ventilation was necessary in order to produce a beneficial effect of iNO on the ventilation-perfusion distribution and oxygenation.[58,59] In patients with ARDS, factors determining NO-induced improvement in arterial oxygenation and pulmonary vascular effects have been shown to be PEEP-induced alveolar recruitment and the baseline level of PVR.[60] This is in some contradiction to the findings of Gommers et al.,[32] who found an improved oxygenation by iNO following surfactant treatment in lung-lavaged rabbits, but not in the group of animals treated with an increase of PEEP-levels to 10cm H2O. No matter what lung recruitment strategy used, the crucial point appears to enhance homogenous alveolar ventilation and to ensure widespread distribution of NO across the lung. This facilitates diffusion of iNO from the alveoli to the pulmonary capillaries and increases the proportion of iNO affecting vascular relaxation of the smooth muscle cell as mediated by the pulmonary endothelium.
6. Conclusion
iNO has been shown to improve oxygenation in neonates with PPHN secondary to a variety of disease entities. Although a reduction of mortality in iNO-treated neonates has not been demonstrated, the number of neonates proceeding to an invasive and morbidity-associated treatment like ECMO was shown to be decreased. This occurred without prolongation of total ventilator treatment or hospital days, and without an increase in the incidence of CLD. In relative numbers this means 1 patient requiring ECMO less for every 6 patients treated with NO.[61] From a clinical as well as economic standpoint this therapeutic option appears promising. Clinically more ambiguous is the situation in the preterm neonate. The majority of these neonates are not candidates for ECMO because of either bodyweight or gestational age criteria, which would favour an early use of iNO. On the other hand, these neonates are at risk of experiencing IVH because of the immaturity of their cerebral vasculature. Although the data of Kinsella[36] and the Franco-Belgium Group[37] indicate safety as related to the incidence of IVH, iNO treatment in preterm neonates remains an experimental therapy as only recently commented on by Saugstad.[62] In spite of all caution, there is no way around the initiation and performance of randomised controlled trials of iNO in preterm neonates in order to answer these remaining questions. The most important aspect of such a study would have to be the question of safety of iNO in preterm neonates with regard to potential interactions between NO-triggered inhibition of platelet aggregation and progression of pre-existing IVHs. Additional aspects of such a trial could be the relationship between response rate and the mode of ventilation or the use of lung recruitment strategies, respectively. The latter include surfactant treatment primarily in preterm neonates but also in term neonates with CDH, lung hypoplasia of other origin and MAS.
Response to iNO irrespective of gestational age appears to be dependent on the predominant disease entity or, more specifically, on the degree of lung expansion associated with certain disease categories. Alveolar recruitment by the use of HFV appears superior to that achieved with conventional ventilation, although this might be ultimately related to the standard settings of ventilatory pressures clinically used (PEEP, MAP). Again there is a need for randomised controlled trials comparing lung recruitment strategies during HFV and conventional ventilation and their effect on the efficacy of iNO. Ventilatory strategies would need to be highly uniform in order to successfully perform such a trial. This will be the real challenge on the way to establish the role of iNO in neonates.
References
Roberts JD, Polaner DM, Lang P, et al. Inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Lancet 1992; 340: 818–9
Kinsella JP, Neish SR, Shaffer E, et al. Low-dose inhalation nitric oxide in persistent pulmonary hypertension of the newborn. Lancet 1992; 340: 819–20
Roberts Jr JD, Fineman JR, Morin 3rd FC, et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn: the Inhaled Nitric Oxide Study Group. N Engl J Med 1997; 336: 605–10
Neonatal Inhaled Nitric Oxide Study Group (NINOS). Inhaled nitric oxide and hypoxic respiratory failure in neonates with congenital diaphragmatic hernia. Pediatrics 1997; 99: 838–45
Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. N Engl J Med 1997; 336: 597–604
Wessel DL, Adatia I, Van Marter LJ, et al. Improved oxygenation in a randomized trial of inhaled nitric oxide for persistent pulmonary hypertension of the newborn. Pediatrics 1997; 100: E7
Subhedar NV, Ryan SW, Shaw NJ. Open randomised controlled trial of inhaled nitric oxide and early dexamethasone in high risk preterm infants. Arch Dis Child Fetal Neonatal Ed 1997; 77: F185–90
Van Meurs KP, Rhine WD, Asselin JM, et al. Response of premature infants with severe respiratory failure to inhaled nitric oxide. Preemie NO Collaborative Group. Pediatr Pulmonol 1997; 24: 319–23
Skimming JW, Bender KA, Hutchison AA, et al. Nitric oxide inhalation in infants with respiratory distress syndrome. J Pediatr 1997; 130: 225–30
Hogman M, Frostell C, Arnberg H, et al. Bleeding time prolongation and NO inhalation. Lancet 1993; 341(8861): 1664–5
Samama CM, Diaby M, Fellahi JL, et al. Inhibition of platelet aggregation by inhaled nitric oxide in patients with acute respiratory distress syndrome. Anesthesiology 1995; 83: 56–65
Cheung PY, Salas E, Etches PC, et al. Inhaled nitric oxide and inhibition of platelet aggregation in critically ill neonates. Lancet 1998; 351: 1181–2
George TN, Johnson KJ, Bates JN, et al. The effect of inhaled nitric oxide therapy on bleeding time and platelet aggregation in neonates. J Pediatr 1998; 132: 731–4
Miller OI, Gaynor JW, Macrae DJ, et al. Inhaled nitric oxide for pulmonary hypertension after repair of exomphalos. Arch Dis Child 1993; 69: 518–20
Schranz D, Huth R, Wippermann CF, et al. Nitric oxide and prostacyclin lower suprasystemic pulmonary hypertension after cardiopulmonary bypass. Eur J Pediatr 1993; 152: 793–6
Journois D, Pouard P, Mauriat P, et al. Inhaled nitric oxide as a therapy for pulmonary hypertension after operations for congenital heart defects. J Thorac Cardiovasc Surg 1994; 107: 1129–35
Karamanoukian HL, Glick PL, Zayek M, et al. Inhaled nitric oxide in congenital hypoplasia of the lungs due to diaphragmatic hernia or oligohydramnios. Pediatrics 1994; 94: 715–8
Hoffman GM, Ross GA, Day SE, et al. Inhaled nitric oxide reduces the utilization of extracorporeal membrane oxygenation in persistent pulmonary hypertension of the newborn. Crit Care Med 1997; 25: 352–9
Day RW, Lynch JM, White KS, et al. Acute response to inhaled nitric oxide in newborns with respiratory failure and pulmonary hypertension. Pediatrics 1996; 98: 698–705
Barefield ES, Karle VA, Phillips 3rd JB, et al. Inhaled nitric oxide in term infants with hypoxemic respiratory failure. J Pediatr 1996; 129: 279–86
Kinsella JP, Truog WE, Walsh WF, et al. Randomized, multicenter trial of inhaled nitric oxide and high-frequency oscillatory ventilation in severe, persistent pulmonary hypertension of the newborn. J Pediatr 1997; 131: 55–62
Davidson D, Barefield ES, Kattwinkel J, et al. Inhaled nitric oxide for the early treatment of persistent pulmonary hypertension of the term newborn: a randomized, double-masked, placebo-controlled, dose-response, multicenter study: the I-NO/PPHN Study Group. Pediatrics 1998; 101: 325–34
Finer NN, Barrington KJ. Nitric oxide in respiratory failure in the newborn infant. Semin Perinatol 1997; 21: 426–40
Finer NN, Barrington KJ. Nitric oxide in respiratory failure in full-term and nearly full-term newborn infants (Cochrane review). Cochrane Library [CDROM and online]. Issue 1. Oxford: The Cochrane Collaboration, 1999: review no. 000399
Goldman AP, Tasker RC, Haworth SG, et al. Four patterns of response to inhaled nitric oxide for persistent pulmonary hypertension of the newborn. Pediatrics 1996; 98: 706–13
Rais Bahrami K, Rivera O, Seale WR, et al. Effect of nitric oxide in meconium aspiration syndrome after treatment with surfactant. Crit Care Med 1997; 25: 1744–7
Mercier JC, Lacaze T, Storme L, et al. Disease-related response to inhaled nitric oxide in newborns with severe hypoxaemic respiratory failure: French Paediatric Study Group of Inhaled NO. Eur J Pediatr 1998; 157: 747–52
Steinhorn RH, Cox PN, Fineman JR, et al. Inhaled nitric oxide enhances oxygenation but not survival in infants with alveolar capillary dysplasia. J Pediatr 1997; 130: 417–22
Adler SM, Clark RH. Premature and term neonates have similar responses to inhaled nitric oxide (iNO) [abstract]. Pediatr Res 1998; 43: 272A
Watchko JF. Persistent pulmonary hypertension in a very low birthweight preterm infant. Clin Pediatr (Phila) 1985; 24: 592–5
Amato M, de Roche B, von Muralt G. Persistent pulmonary hypertension in preterm-infants. Padiatr Padol 1986; 21(1): 25–30
Gommers D, Hartog A, van ’t Veen A, et al. Improved oxygenation by nitric oxide is enhanced by prior lung reaeration with surfactant, rather than positive end-expiratory pressure, in lung-lavaged rabbits. Crit Care Med 1997; 25: 1868–73
Peliowski A, Finer NN, Etches PC, et al. Inhaled nitric oxide for premature infants after prolonged rupture of the membranes. J Pediatr 1995; 126: 450–3
Banks BA, Seri I, Ischiropoulos H, et al. Changes in oxygenation with inhaled nitric oxide in severe bronchopulmonary dysplasia. Pediatrics 1999; 103: 610–8
Cheung PY, Peliowski A, Robertson CM. The outcome of very low birth weight neonates. J Pediatr 1998; 133: 735–9
Kinsella JP, Walsh FW, Bose CL, et al. Inhaled nitric oxide in premature neonates with severe hypoxaemic respiratory failure: a randomised controlled trial. Lancet 1999; 354: 1061–5
Franco-Belgium Collaborative NO Trial Group. Early compared with delayed inhaled nitric oxide in moderately hypoxaemic neonates with respiratory failure: a randomised controlled trial. Lancet 1999; 354: 1066–71
Barrington KJ, Finer NN. Inhaled nitric oxide in preterm newborn infants with respiratory failure (Cochrane review). Cochrane Library [CDROM and Online]. Issue 1. Oxford: The Cochrane Collaboration, 1999: review no. 000509
Chang LY, Graham JA, Miller FJ, et al. Effects of subchronic inhalation of low concentrations of nitrogen dioxide: the proximal alveolar region of juvenile and adult rats. Toxicol Appl Pharmacol 1986; 83: 45–61
Hallman M, Bry K, Turbow R, et al. Pulmonary toxicity associated with nitric oxide in term infants with severe respiratory failure. J Pediatr 1998; 132: 827–9
Hoehn T, Huebner J, Paboura E, et al. Effect of therapeutic concentrations of nitric oxide on bacterial growth in vitro. Crit Care Med 1998; 26: 1857–62
Christou H, Magnani B, Morse DS, et al. Inhaled nitric oxide does not affect adenosine 5′-diphosphate-dependent platelet activation in infants with persistent pulmonary hypertension of the newborn. Pediatrics 1998; 102: 1390–3
Crow JP, Beckman JS. The role of peroxynitrite in nitric oxide-mediated toxicity. Curr Top Microbiol Immunol 1995; 196: 57–73
Gries A, Bode C, Peter K, et al. Inhaled nitric oxide inhibits human platelet aggregation, P-selectin expression, and fibrinogen binding invitroand invivo. Circulation 1998; 97(15): 1481–7
Varela AF, Runge A, Ignarro LJ, et al. Nitric oxide and prostacyclin inhibit fetal platelet aggregation: a response similar to that observed in adults. Am J Obstet Gynecol 1992; 167: 1599–604
Bassenge E. Antiplatelet effects of endothelium-derived relaxing factor and nitric oxide donors. Eur Heart J 1991; 12 Suppl. E: 12–5
Huang QW, Sun B, Gao F, et al. Effects of inhaled nitric oxide and high-frequency ventilation in rabbits with meconium aspiration. Biol Neonate 1999; 76: 374–82
Gessler P, Nebe T, Birle A, et al. A new side effect of inhaled nitric oxide in neonates and infants with pulmonary hypertension: functional impairment of the neutrophil respiratory burst. Intens Care Med 1996; 22: 252–8
Iha S, Orita K, Kanno T, et al. Oxygen-dependent inhibition of neutrophil respiratory burst by nitric oxide. Free Radic Res 1996; 25: 489–98
Hogman M, Frosteil CG, Hedenstrom H, et al. Inhalation of nitric oxide modulates adult human bronchial tone. Am Rev Respir Dis 1993; 148: 1474–8
Hogman M, Frostell C, Arnberg H, et al. Inhalation of nitric oxide modulates methacholine-induced bronchoconstriction in the rabbit. Eur Respir J 1993; 6: 177–80
Dupuy PM, Shore SA, Drazen JM, et al. Bronchodilator action of inhaled nitric oxide in guinea pigs. J Clin Invest 1992; 90: 421–8
Folkerts G, van der Linde HJ, Nijkamp FP. Virus-induced airway hyperresponsiveness in guinea pigs is related to a deficiency in nitric oxide. J Clin Invest 1995; 95: 26–30
Kinsella JP, Parker TA, Galan H, et al. Effects of inhaled nitric oxide on pulmonary edema and lung neutrophil accumulation in severe experimental hyaline membrane disease. Pediatr Res 1997; 41: 457–63
Abman SH, Kinsella JP. Inhaled nitric oxide for persistent pulmonary hypertension of the newborn: the physiology matters! Pediatrics 1995; 96: 1153–5
Hoehn T, Krause M, Hentschel R. High-frequency ventilation augments the effect of inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Eur Respir J 1998; 11: 234–8
Hoehn T, Krause MF. Synergistic effects of high-frequency ventilation and inhaled nitric oxide in the treatment of hypoxemic respiratory failure in infancy. Pediatr Pulmonol 1998; 26: 228–30
Putensen C, Rasanen J, Downs JB. Effect of endogenous and inhaled nitric oxide on the ventilation-perfusion relationships in oleic-acid lung injury. Am J Respir Crit Care Med 1994; 150: 330–6
Putensen C, Rasanen J, Lopez FA, et al. Continuous positive airway pressure modulates effect of inhaled nitric oxide on the ventilation-perfusion distributions in canine lung injury. Chest 1994; 106: 1563–9
Puybasset L, Rouby JJ, Mourgeon E, et al. Factors influencing cardiopulmonary effects of inhaled nitric oxide in acute respiratory failure. Am J Respir Crit Care Med 1995; 152: 318–28
Truog WE. Inhaled nitric oxide: a tenth anniversary observation. Pediatrics 1998; 101: 696–7
Saugstad OD. Inhaled nitric oxide for preterm infants: still an experimental therapy. Lancet 1999; 354: 1047–8
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoehn, T., Krause, M.F. Response to Inhaled Nitric Oxide in Premature and Term Neonates. Drugs 61, 27–39 (2001). https://doi.org/10.2165/00003495-200161010-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200161010-00004