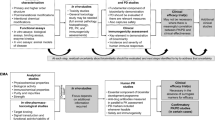
Abstract
The potential benefits of early patient access to new medicines in areas of high unmet medical need are recognised, but uncertainties concerning effectiveness, safety and added value when new medicines are authorised, and subsequently funded based on initial preliminary data only, have important implications. In 2016 olaratumab received accelerated conditional approval from both the European Medicines Agency and the US Food and Drug Administration for the treatment of soft-tissue sarcoma, based on the claims of a substantial reduction in the risk of death with an 11.8-month improvement in median overall survival in a phase II trial in combination with doxorubicin vs. doxorubicin alone. The failure to confirm these benefits in the post-authorisation pivotal trial has highlighted key concerns regarding early access and conditional approvals for new medicines. Concerns include potentially considerable clinical and economic costs, so that patients may have received suboptimal treatment and any money spent has foregone the opportunity to improve access to effective treatments. As a result, it seems reasonable to reconsider current marketing authorisation models and approaches. Potential pathways forward include closer collaboration between regulators, pharmaceutical companies and payers to enhance the generation of rapid and comparative confirmatory trials in a safe and fair manner, with minimal patient exposure as required to achieve robust evidence. Additionally, it may be time to review early access systems, and to explore new avenues regarding who should pay or part pay for new treatments whilst information is being collected as part of any obligations for conditional marketing authorisation. Greater co-operation between countries regarding the collection of data in routine clinical care, and further research on post-marketing data analysis and interpretation, may also contribute to improved appraisal and continued access to new innovative cancer treatments.
Similar content being viewed by others
References
OECD. Health at a glance 2017: OECD indicators. [Internet]. https://www.oecd-ilibrary.org/social-issues-migration-health/health-at-a-glance-2017_health_glance-2017-en. Accessed 03 Nov 2019.
Arzneiverordnungs-Report 2018. Schwabe U, Paffrath D, Ludwig W-D, Klauber J, editors. https://www.bookdepository.com/Arzneiverordnungs-Report-2018-Ulrich-Schwabe/9783662573853. Accessed 07 Oct 2019.
Godman B, Malmstrom RE, Diogene E, Gray A, Jayathissa S, Timoney A, et al. Are new models needed to optimize the utilization of new medicines to sustain healthcare systems? Expert Rev Clin Pharmacol. 2015;8(1):77–94.
Malmstrom RE, Godman BB, Diogene E, Baumgartel C, Bennie M, Bishop I, et al. Dabigatran: a case history demonstrating the need for comprehensive approaches to optimize the use of new drugs. Front Pharmacol. 2013;4:39.
Godman B, Bucsics A, Vella Bonanno P, Oortwijn W, Rothe CC, Ferrario A, et al. Barriers for access to new medicines: searching for the balance between rising costs and limited budgets. Front Public Health. 2018;6:328.
WHO. Access to new medicines in Europe: technical review of policy initiatives and opportunities for collaboration and research. http://www.euro.who.int/__data/assets/pdf_file/0008/306179/Access-new-medicines-TR-PIO-collaboration-research.pdf?ua=1. Accessed 07 Oct 2019.
Kelly RJ, Smith TJ. Delivering maximum clinical benefit at an affordable price: engaging stakeholders in cancer care. Lancet Oncol. 2014;15(3):e112–8.
Chalkidou K, Marquez P, Dhillon PK, Teerawattananon Y, Anothaisintawee T, Gadelha CA, et al. Evidence-informed frameworks for cost-effective cancer care and prevention in low, middle, and high-income countries. Lancet Oncol. 2014;15(3):e119–31.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Howard DH, Bach P, Berndt ER, Conti RM. Pricing in the market for anticancer drugs. J Econ Perspect. 2015;29(1):139–62.
Godman B, Wild C, Haycox A. Patent expiry and costs for anti-cancer medicines for clinical use. GaBI J. 2017;6(3):105–6.
Kantarjian HM, Fojo T, Mathisen M, Zwelling LA. Cancer drugs in the United States: justum pretium - the just price. J Clin Oncol. 2013;31(28):3600–4.
Cohen D. Cancer drugs: high price, uncertain value. BMJ. 2017;359:j4543.
Grössmann N, Wild C. Between January 2009 and April 2016, 134 novel anticancer therapies were approved: what is the level of knowledge concerning the clinical benefit at the time of approval? ESMO Open. 2017;1:e000125. https://doi.org/10.1136/esmoopen-2016-000125.
Dusetzina SB. Drug pricing trends for orally administered anticancer medications reimbursed by commercial health plans, 2000-2014. JAMA Oncol. 2016;2(7):960–1.
Haycox A. Why cancer? Pharmacoeconomics. 2016;34(7):625–7.
Gyawali B, Sullivan R. Economics of cancer medicines: for whose benefit? New Bioeth. 2017;23(1):95–104.
Luzzatto L, Hyry HI, Schieppati A, Costa E, Simoens S, Schaefer F, et al. Outrageous prices of orphan drugs: a call for collaboration. Lancet. 2018;392(10149):791–4.
Godman B, Oortwijn W, de Waure C, Mosca I, Puggina A, Specchia ML, et al. Links between pharmaceutical R&D models and access to affordable medicines: a study for the ENVI Committee. Available from: http://www.europarl.europa.eu/RegData/etudes/STUD/2016/587321/IPOL_STU(2016)587321_EN.pdf. Accessed 7 Oct 2019.
Simoens S, Picavet E, Dooms M, Cassiman D, Morel T. Cost-effectiveness assessment of orphan drugs: a scientific and political conundrum. Appl Health Econ Health Policy. 2013;11(1):1–3.
Cohen JP, Felix A. Are payers treating orphan drugs differently? J Mark Access Health Policy. 2014;15:2. https://doi.org/10.3402/jmahp.v2.23513.
Godman B, Campbell S, Suh HS, Finlayson AE, Bennie M, Gustafsson LL. Ongoing measures to enhance prescribing efficiency across Europe: implications for other countries. J Health Tech Assess. 2013;1(27–42). [Internet]. https://core.ac.uk/download/pdf/131165241.pdf. Accessed 03 Nov 2019.
Dolgin E. Bringing down the cost of cancer treatment. Nature. 2018;555(7695):S26–9.
IQVIA Institute for Human Data Science. Global oncology trends 2018. https://www.iqvia.com/institute/reports/global-oncology-trends-2018. Accessed 07 Oct 2019.
IMS Institute for Healthcare Informatics. Global oncology trend report: a review of 2015 and outlook to 2020. June 2016. Available from: https://www.scribd.com/document/323179495/IMSH-Institute-Global-Oncology-Trend-2015-2020-Report. Accessed 7 Oct 2019.
Lancet The. GLOBOCAN 2018: counting the toll of cancer. Lancet. 2018;392(10152):985.
Durkee BY, Qian Y, Pollom EL, King MT, Dudley SA, Shaffer JL, et al. Cost-effectiveness of pertuzumab in human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2016;34(9):902–9.
Hoen Et. Access to cancer treatment: a study of medicine pricing issues with recommendations for improving access to cancer medication. http://apps.who.int/medicinedocs/documents/s21758en/s21758en.pdf. Accessed 07 Oct 2019.
Hill A, Gotham D, Fortunak J, Meldrum J, Erbacher I, Martin M, et al. Target prices for mass production of tyrosine kinase inhibitors for global cancer treatment. BMJ Open. 2016;6(1):e009586.
Hill A, Redd C, Gotham D, Erbacher I, Meldrum J, Harada R. Estimated generic prices of cancer medicines deemed cost-ineffective in England: a cost estimation analysis. BMJ Open. 2017;7(1):e011965.
WHO Fair Pricing. Informal advisory group meeting. WHO Headquarters, Geneva 22-24 November 2016. https://www.who.int/medicines/access/fair_pricing/report_fair_pricing-forumIGmeeting.pdf?ua=1. Accessed 07 Oct 2019.
Barber M, Gotham D, Hill A. Potential price reductions for cancer medicines on the WHO essential medicines list. http://apps.who.int/medicinedocs/documents/s23154en/s23154en.pdf. Accessed 07 Oct 2019.
Campbell JD, Kalo Z. Fair global drug pricing. Expert Rev Pharmacoecon Outcomes Res. 2018;18(6):581–3.
Vian T, Kohler JC, Forte G, Dimancesco D. Promoting transparency, accountability, and access through a multi-stakeholder initiative: lessons from the medicines transparency alliance. J Pharm Policy Pract. 2017;10:18.
Wilking N, Lopes G, Meier K, Simoens S, van Harten W, Vulto A. Can we continue to afford access to cancer treatment? Eur Oncol Haematol. 2017;13(2):114–9.
Sagonowsky E. AbbVie’s massive Humira discounts are stifling Netherlands biosimilars: report. 2019. https://www.fiercepharma.com/pharma/abbvie-stifling-humira-biosim-competition-massive-discounting-dutch-report. Accessed 07 Oct 2019.
Kaplan W, Wirtz V, Mantel-Teeuwisse A, Stolk P, Duthey P, Laing R. Priority medicines for Europe and the world. 2013 update. http://www.who.int/medicines/areas/priority_medicines/MasterDocJune28_FINAL_Web.pdf. Accessed 07 Oct 2019.
Makady A, van Veelen A, de Boer A, Hillege H, Klungel OH, Goettsch W. Implementing managed entry agreements in practice: the Dutch reality check. Health Policy. 2019;123(3):267–74.
Banzi R, Gerardi C, Bertele V, Garattini S. Approvals of drugs with uncertain benefit-risk profiles in Europe. Eur J Intern Med. 2015;26(8):572–84.
Hawkes N. Specialists attack drug agency’s fast track approval scheme. BMJ. 2016;353:i3060.
Ermisch M, Bucsics A, Vella Bonanno P, Arickx F, Bybau A, Bochenek T, et al. Payers’ views of the changes arising through the possible adoption of adaptive pathways. Front Pharmacol. 2016;7:305.
Scavone C, di Mauro G, Mascolo A, Berrino L, Rossi F, Capuano A. The new paradigms in clinical research: from early access programs to the novel therapeutic approaches for unmet medical needs. Front Pharmacol. 2019;10:111.
Leyens L, Brand A. Early patient access to medicines: health technology assessment bodies need to catch up with new marketing authorization methods. Public Health Genomics. 2016;19(3):187–91.
Cole A, Cubi-Molla P, Pollard J, Sim D, Sullivan R, Sussex J, Lorgelly P. Making outcome-based payment a reality in the NHS. [Internet]. https://www.ohe.org/publications/making-outcome-based-payment-reality-nhs. Accessed 03 Nov 2019.
European Medicines Agency. PRIME: priority medicines. 2019. https://www.ema.europa.eu/en/human-regulatory/research-development/prime-priority-medicines. Accessed 07 Oct 2019.
European Medicines Agency. Adaptive pathways. [Internet]. https://www.ema.europa.eu/en/human-regulatory/research-development/adaptive-pathways. Accessed 03 Nov 2019.
Grignolo A, Pretorius S. Phase III trial failures, costly but preventable. Appl Clin Trials. 2016;25(8). [Internet]. http://www.appliedclinicaltrialsonline.com/phase-iii-trial-failures-costly-preventable. Accessed 03 Nov 2019.
Amiri-Kordestani L, Fojo T. Why do phase III clinical trials in oncology fail so often? J Natl Cancer Inst. 2012;104(8):568–9.
Gyawali B, Addeo A. Negative phase 3 randomized controlled trials: why cancer drugs fail the last barrier? Int J Cancer. 2018;143(8):2079–81.
Walter RB, Appelbaum FR, Tallman MS, Weiss NS, Larson RA, Estey EH. Shortcomings in the clinical evaluation of new drugs: acute myeloid leukemia as paradigm. Blood. 2010;116(14):2420–8.
Jonker DJ, Nott L, Yoshino T, Gill S, Shapiro J, Ohtsu A, et al. Napabucasin versus placebo in refractory advanced colorectal cancer: a randomised phase 3 trial. Lancet Gastroenterol Hepatol. 2018;3(4):263–70.
Prasad V, De Jesus K, Mailankody S. The high price of anticancer drugs: origins, implications, barriers, solutions. Nat Rev Clin Oncol. 2017;14(6):381–90.
Joppi R, Cinconze E, Mezzalira L, Pase D, Poggiani C, Rossi E, et al. Hospitalized patients with atrial fibrillation compared to those included in recent trials on novel oral anticoagulants: a population-based study. Eur J Intern Med. 2013;24(4):318–23.
Bouvy JC, Sapede C, Garner S. Managed entry agreements for pharmaceuticals in the context of adaptive pathways in Europe. Front Pharmacol. 2018;9:280.
Ioannidis JPA. What have we (not) learnt from millions of scientific papers with P values? Am Stat. 2019;73(Suppl. 1):20–5.
Godman B, Wettermark B, van Woerkom M, Fraeyman J, Alvarez-Madrazo S, Berg C, et al. Multiple policies to enhance prescribing efficiency for established medicines in Europe with a particular focus on demand-side measures: findings and future implications. Front Pharmacol. 2014;5:106.
Moorkens E, Vulto AG, Huys I, Dylst P, Godman B, Keuerleber S, et al. Policies for biosimilar uptake in Europe: an overview. PloS One. 2017;12(12):e0190147.
Dylst P, Vulto A, Simoens S. Demand-side policies to encourage the use of generic medicines: an overview. Expert Rev Pharmacoecon Outcomes Res. 2013;13(1):59–72.
Matusewicz W, Godman B, Pedersen HB, Furst J, Gulbinovic J, Mack A, et al. Improving the managed introduction of new medicines: sharing experiences to aid authorities across Europe. Expert Rev Pharmacoecon Outcomes Res. 2015;15(5):755–8.
Godman B, Malmstrom RE, Diogene E, Jayathissa S, McTaggart S, Cars T, et al. Dabigatran: a continuing exemplar case history demonstrating the need for comprehensive models to optimize the utilization of new drugs. Front Pharmacol. 2014;5:109.
Eriksson I, Wettermark B, Persson M, Edstrom M, Godman B, Lindhe A, et al. The early awareness and alert system in Sweden: history and current status. Front Pharmacol. 2017;8:674.
Wettermark B, Godman B, Eriksson C, van Ganse E, Garattini S, Joppi R, et al. Einführung neuer Arzneimittel in europäische Gesundheitssysteme (Introduction of new medicines into European healthcare systems). GGW. 2010;10(3):24–34. [Internet]. https://www.wido.de/fileadmin/Dateien/Dokumente/Publikationen_Produkte/GGW/wido_ggw_0310_wettermark_et_al.pdf. Accessed 03 Nov 2019.
Kemp-Casey A, Pratt N, Ramsay E, Roughead EE. Using post-market utilisation analysis to support medicines pricing policy: an Australian case study of aflibercept and ranibizumab use. Appl Health Econ Health Policy. 2019;17(3):411–7.
Garrison LP, Towse A. Value-based pricing and reimbursement in personalised healthcare: introduction to the basic health economics. J Pers Med. 2017;7(3):10.
Garner S, Rintoul A, Hill SR. Value-based pricing: L’enfant terrible? Pharmacoeconomics. 2018;36(1):5–6.
MOCA. Process on corporate social responsibility in the field of pharmaceuticals platform on access to medicines in Europe Working Group on Mechanism of Coordinated Access to Orphan Medicinal Products (MoCA-OMP). http://download2.eurordis.org.s3.amazonaws.com/moca/history/WG%20MoCA-OMP%20Transparent%20Value%20Framework.pdf. Accessed 07 Oct 2019.
Seixas BV, Dionne F, Conte T, Mitton C. Assessing value in health care: using an interpretive classification system to understand existing practices based on a systematic review. BMC Health Serv Res. 2019;19(1):560.
Ferrario A, Kanavos P. Managed entry agreements for pharmaceuticals: the European experience. EMiNet, Brussels, Belgium. 2013. http://eprints.lse.ac.uk/50513/. Accessed 07 Oct 2019.
Ferrario A, Arāja D, Bochenek T, Čatić T, Dankó D, Dimitrova M, et al. The implementation of managed entry agreements in central and Eastern Europe: findings and implications. Pharmacoeconomics. 2017;35(12):1271–85.
Pauwels K, Huys I, Vogler S, Casteels M, Simoens S. Managed entry agreements for oncology drugs: lessons from the European experience to inform the future. Front Pharmacol. 2017;8:171.
Carlson JJ, Chen S, Garrison LP Jr. Performance-based risk-sharing arrangements: an updated international review. Pharmacoeconomics. 2017;35(10):1063–72.
Piatkiewicz TJ, Traulsen JM, Holm-Larsen T. Risk-sharing agreements in the EU: a systematic review of major trends. Pharmacoecon Open. 2018;2(2):109–23.
Nazareth T, Ko JJ, Sasane R, Frois C, Carpenter S, Demean S, et al. Outcomes-based contracting experience: research findings from US and European stakeholders. J Manag Care Spec Pharm. 2017;23(10):1018–26.
Morel T, Arickx F, Befrits G, Siviero P, van der Meijden C, Xoxi E, et al. Reconciling uncertainty of costs and outcomes with the need for access to orphan medicinal products: a comparative study of managed entry agreements across seven European countries. Orphanet J Rare Dis. 2013;8:198.
MAP BioPharm. AIFA announces funding of CAR-T cell therapy using new reimbursement approach. 2019. https://mapbiopharma.com/2019/08/aifa-announces-funding-of-car-t-cell-therapy-using-new-reimbursement-approach/. Accessed 07 Oct 2019.
Ferguson JS, Summerhayes M, Masters S, Schey S, Smith IE. New treatments for advanced cancer: an approach to prioritization. Br J Cancer. 2000;83(10):1268–73.
Wild C, Grossmann N, Bonanno PV, Bucsics A, Furst J, Garuoliene K, et al. Utilisation of the ESMO-MCBS in practice of HTA. Ann Oncol. 2016;27(11):2134–6.
Lopes G, Vulto A, Wilking N, van Harten W, Meier K, Simoens S. Potential solutions for sustaining the costs of cancer drugs. Eur Oncol Haematol. 2017;13(2):102–7.
Garattini L, Curto A. Performance-based agreements in Italy: ‘trendy outcomes’ or mere illusions? Pharmacoeconomics. 2016;34(10):967–9.
Goble JA, Ung B, van Boemmel-Wegmann S, Navarro RP, Parece A. Performance-based risk-sharing arrangements: US payer experience. J Manag Care Spec Pharm. 2017;23(10):1042–52.
Yu JS, Chin L, Oh J, Farias J. Performance-based risk-sharing arrangements for pharmaceutical products in the United States: a systematic review. J Manag Care Spec Pharm. 2017;23(10):1028–40.
Makady A, van Acker S, Nijmeijer H, de Boer A, Hillege H, Klungel O, et al. Conditional financing of drugs in the Netherlands: past, present, and future: results from stakeholder interviews. Value Health. 2019;22(4):399–407.
Davis C, Lexchin J, Jefferson T, Gotzsche P, McKee M. “Adaptive pathways” to drug authorisation: adapting to industry? BMJ. 2016;354:i4437.
Garattini L, Curto A, van de Vooren K. Do the current performance-based schemes in Italy really work? “Success fee”: a novel measure for cost-containment of drug expenditure. Value Health. 2015;18(2):352.
Navarria A, Drago V, Gozzo L, Longo L, Mansueto S, Pignataro G, et al. Do the current performance-based schemes in Italy really work? “Success fee”: a novel measure for cost-containment of drug expenditure. Value Health. 2015;18(1):131–6.
Columbus G. Olaratumab combination misses OS endpoint for sarcoma in phase III trial. 2019. https://www.targetedonc.com/news/olaratumab-combination-misses-os-endpoint-for-sarcoma-in-phase-iii-trial. Accessed 07 Oct 2019.
Lilly USA. Lilly reports results of phase 3 soft tissue sarcoma study of LARTRUVO®. 18 Jan 2019. Available from: https://investor.lilly.com/news-releases/news-release-details/lilly-reports-results-phase-3-soft-tissue-sarcoma-study. Accessed 07 Oct 2019.
Vella Bonanno P, Ermisch M, Godman B, Martin AP, Van Den Bergh J, Bezmelnitsyna L, et al. Adaptive pathways: possible next steps for payers in preparation for their potential implementation. Front Pharmacol. 2017;8:497.
Lilly. Lartruvo: summary of product characteristics. https://www.ema.europa.eu/documents/product-information/lartruvo-epar-product-information_en.pdf. Accessed 07 Oct 2019.
Tap WD, Jones RL, Van Tine BA, Chmielowski B, Elias AD, Adkins D, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388(10043):488–97.
EMA. EPAR Lartruvo: international non-proprietary name: olaratumab: assessment report. September 2016. https://www.ema.europa.eu/en/documents/assessment-report/lartruvo-epar-public-assessment-report_en.pdf. Accessed 07 Oct 2019.
European Medicines Agency. Lartruvo. 2016. https://www.ema.europa.eu/en/documents/overview/lartruvo-epar-summary-public_en.pdf. Accessed 07 Oct 2019.
Food and Drug Administration. FDA news release: FDA grants accelerated approval to new treatment for advanced soft tissue sarcoma. 2016. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm525878.htm. Accessed 07 Oct 2019.
Tap WD, Wagner AJ, Papai Z, Ganjoo KN, Yen C-C, Schoffski P, et al. ANNOUNCE: a randomized, placebo (PBO)-controlled, double-blind, phase (Ph) III trial of doxorubicin (dox) + olaratumab versus dox + PBO in patients (pts) with advanced soft tissue sarcomas (STS). J Clin Oncol. 2019;37(18_Suppl.):LBA3-LBA. https://ascopubs.org/doi/abs/10.1200/JCO.2019.37.18_suppl.LBA3. Accessed 03 Nov 2019.
European Medicines Agency. No new patients should start treatment with Lartruvo after study shows cancer medicine does not prolong life. 2019. https://www.ema.europa.eu/en/news/no-new-patients-should-start-treatment-lartruvo-after-study-shows-cancer-medicine-does-not-prolong. Accessed 07 Oct 2019.
US Food and Drug Administration. FDA news release: FDA grants accelerated approval to new treatment for advanced soft tissue sarcoma. 2019. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm525878.htm. Accessed 07 Oct 2019.
Eichler HG, Oye K, Baird LG, Abadie E, Brown J, Drum CL, et al. Adaptive licensing: taking the next step in the evolution of drug approval. Clin Pharmacol Ther. 2012;91(3):426–37.
Baird LG, Banken R, Eichler HG, Kristensen FB, Lee DK, Lim JC, et al. Accelerated access to innovative medicines for patients in need. Clin Pharmacol Ther. 2014;96(5):559–71.
Bouvy JC, Jonsson P, Longson C, Crabb N, Garner S. Health technology assessment in the context of adaptive pathways for medicines in Europe: challenges and opportunities. Clin Pharmacol Ther. 2016;100(6):594–7.
Barrett A, Roques T, Small M, Smith RD. How much will Herceptin really cost? BMJ. 2006;333(7578):1118–20.
Pontes C, Fontanet JM, Vives R, Sancho A, Gomez-Valent M, Rios J, et al. Evidence supporting regulatory-decision making on orphan medicinal products authorisation in Europe: methodological uncertainties. Orphanet J Rare Dis. 2018;13(1):206.
Godman B, Finlayson AE, Cheema PK, Zebedin-Brandl E, Gutierrez-Ibarluzea I, Jones J, et al. Personalizing health care: feasibility and future implications. BMC Med. 2013;11:179.
US FDA. FDA approves larotrectinib for solid tumors with NTRK gene fusions. 2018. https://www.fda.gov/drugs/fda-approves-larotrectinib-solid-tumors-ntrk-gene-fusions-0. Accessed 07 Oct 2019.
Davis C, Naci H, Gurpinar E, Poplavska E, Pinto A, Aggarwal A. Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: retrospective cohort study of drug approvals 2009–2013. BMJ. 2017;359:j4530.
European Medicines Agency. Conditional marketing authorisation. EMA/471951/2016. https://www.ema.europa.eu/documents/report/conditional-marketing-authorisation-report-ten-years-experience-european-medicines-agency_en.pdf. Accessed 07 Oct 2019.
Tao D, Schott S, Prasad V. The tradeoff of cancer drug regulatory policy: faster approvals for one means less knowledge for another. Am J Med. 2019;132(3):e509–11.
Editorial. New 50 million pound cancer fund already intellectually bankrupt. Lancet. 2010;376(9739):389.
European Medicines Agency. Points to consider on application with 1. Meta-analyses; 2. One pivotal study. CPMP/EWP/2330/99. 2001. https://www.ema.europa.eu/en/application-1-meta-analyses-2-one-pivotal-study. Accessed 07 Oct 2019.
Hemmings R. An overview of statistical and regulatory issues in the planning, analysis, and interpretation of subgroup analyses in confirmatory clinical trials. J Biopharm Stats. 2014;24(1):4–18.
Frisk P, Aggefors K, Cars T, Feltelius N, Loov SA, Wettermark B, et al. Introduction of the second-generation direct-acting antivirals (DAAs) in chronic hepatitis C: a register-based study in Sweden. Eur J Clin Pharmacol. 2018;74(7):971–8.
Mueller T, Alvarez-Madrazo S, Robertson C, Wu O, Bennie M. Comparative safety and effectiveness of direct oral anticoagulants in patients with atrial fibrillation in clinical practice in Scotland. Br J Clin Pharmacol. 2019;85(2):422–31.
Xoxi E, Tomino C, De Nigro L, Pani L. The Italian post-marketing registries. Pharm. Program. 2012;5:57–60.
Wallach JD, Ciani O, Pease AM, Gonsalves GS, Krumholz HM, Taylor RS, et al. Comparison of treatment effect sizes from pivotal and postapproval trials of novel therapeutics approved by the FDA based on surrogate markers of disease: a meta-epidemiological study. BMC Med. 2018;16(1):45.
Zeitoun JD, Baron G, Vivot A, Atal I, Downing NS, Ross JS, et al. Post-marketing research and its outcome for novel anticancer agents approved by both the FDA and EMA between 2005 and 2010: a cross-sectional study. Int J Cancer. 2018;142(2):414–23.
KCE. How to improve the Belgian process for managed entry agreements? 2017. https://kce.fgov.be/sites/default/files/atoms/files/Download%20the%20synthesis%20in%20English%20%2840%20p.%29.pdf. Accessed 07 Oct 2019.
European Commission. Innovative payment models for high-cost innovative medicines. https://ec.europa.eu/health/expert_panel/sites/expertpanel/files/docsdir/opinion_innovative_medicines_en.pdf. Accessed 07 Oct 2019.
European Commission. Council Directive 89/105/EEC of 21 December 1988 relating to the transparency of measures regulating the pricing of medicinaI products for human use and their inclusion in the scope of national health insurance systems. 1989. https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-1/dir_1989_105/dir_1989_105_en.pdf. Accessed 07 Oct 2019.
Vogler S, Paris V, Ferrario A, Wirtz VJ, de Joncheere K, Schneider P, et al. How can pricing and reimbursement policies improve affordable access to medicines? Lessons learned from European countries. Appl Health Econ Health Policy. 2017;15(3):307–21.
Vogler S, Paris V, Panteli D. European observatory policy briefs. In: Richardson E, Palm W, Mossialos E, editors. Ensuring access to medicines: how to redesign pricing, reimbursement and procurement? Copenhagen: World Health Organization (acting as the host organization for, and secretariat of, the European Observatory on Health Systems and Policies); 2018.
UN News. Greater transparency, fairer prices for medicines ‘a global human rights issue’, says UN health agency. 2019. https://news.un.org/en/story/2019/04/1036651. Accessed 07 Oct 2019.
United Nations. United Nations Secretary-General’s High-Level Panel on Access to Medicine. 2016. https://static1.squarespace.com/static/562094dee4b0d00c1a3ef761/t/57d9c6ebf5e231b2f02cd3d4/1473890031320/UNSG+HLP+Report+FINAL+12+Sept+2016.pdf. Accessed 07 Oct 2019.
EFPIA. Value of medicines. Section 4: why are the net prices of medicines not more transparent? 2019. https://www.efpia.eu/about-medicines/use-of-medicines/value-of-medicines/. Accessed 07 Oct 2019.
Turajlic S, Sottoriva A, Graham T, Swanton C. Resolving genetic heterogeneity in cancer. Nat Rev Genet. 2019;20(7):404–16.
Antonanzas F, Rodriguez-Ibeas R, Juarez-Castello CA. Personalized medicine and pay for performance: should pharmaceutical firms be fully penalized when treatment fails? Pharmacoeconomics. 2018;36(7):733–43.
Kesselheim AS, Myers JA, Solomon DH, Winkelmayer WC, Levin R, Avorn J. The prevalence and cost of unapproved uses of top-selling orphan drugs. PLoS One. 2012;7(2):e31894.
The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts. Blood. 2013;121(22):4439–42.
Pegram MD, Bondarenko I, Zorzetto MMC, Hingmire S, Iwase H, Krivorotko PV, et al. PF-05280014 (a trastuzumab biosimilar) plus paclitaxel compared with reference trastuzumab plus paclitaxel for HER2-positive metastatic breast cancer: a randomised, double-blind study. Br J Cancer. 2019;120(2):172–82.
Taylor P. Herceptin. FiercePharma. 2017. https://www.fiercepharma.com/special-report/herceptin-2. Accessed 07 Oct 2019.
Genentech. Herceptin: trastuzumab. Prescribing information. 2019. https://www.herceptin.com/hcp/. Accessed 07 Oct 2019.
Workman P, Draetta GF, Schellens JH, Bernards R. How much longer will we put up with $100,000 cancer drugs? Cell. 2017;168(4):579–83.
KEI. Delinkage. http://delinkage.org/overview/. Accessed 07 Oct 2019.
Outterson K, Gopinathan U, Clift C, So AD, Morel CM, Rottingen JA. Delinking investment in antibiotic research and development from sales revenues: the challenges of transforming a promising idea into reality. PLoS Med. 2016;13(6):e1002043.
Raaschou P, Simard JF, Asker Hagelberg C, Askling J. Rheumatoid arthritis, anti-tumour necrosis factor treatment, and risk of squamous cell and basal cell skin cancer: cohort study based on nationwide prospectively recorded data from Sweden. BMJ. 2016;352:i262.
Iannone F, Gremese E, Atzeni F, Biasi D, Botsios C, Cipriani P, et al. Longterm retention of tumor necrosis factor-alpha inhibitor therapy in a large italian cohort of patients with rheumatoid arthritis from the GISEA registry: an appraisal of predictors. J Rheumatol. 2012;39(6):1179–84.
Garcia-Doval I, Cohen AD, Cazzaniga S, Feldhamer I, Addis A, Carretero G, et al. Risk of serious infections, cutaneous bacterial infections, and granulomatous infections in patients with psoriasis treated with anti-tumor necrosis factor agents versus classic therapies: prospective meta-analysis of Psonet registries. J Am Acad Dermatol. 2017;76(2):299–308.e16.
Dos Santos JB, Almeida AM, Acurcio FA, de Oliveira Junior HA, Kakehasi AM, Guerra Junior AA, et al. Comparative effectiveness of adalimumab and etanercept for rheumatoid arthritis in the Brazilian Public Health System. J Comp Eff Res. 2016;5(6):539–49.
Blin P, Dureau-Pournin C, Cottin Y, Benichou J, Mismetti P, Abouelfath A, et al. Comparative effectiveness and safety of standard or reduced dose dabigatran vs. rivaroxaban in non-valvular atrial fibrillation. Clin Pharmacol Ther. 2019;105(6):1439–55.
Maura G, Pariente A, Alla F, Billionnet C. Adherence with direct oral anticoagulants in nonvalvular atrial fibrillation new users and associated factors: a French nationwide cohort study. Pharmacoepidemiol Drug Saf. 2017;26(11):1367–77.
BertelsmannStiftung. SmartHealthSystems International comparison of digital strategies. 2018. https://www.bertelsmann-stiftung.de/fileadmin/files/Projekte/Der_digitale_Patient/VV_SHS-Studie_EN.pdf. Accessed 07 Oct 2019.
Pearson SD, Dreitlein WB, Towse A, Hampson G, Henshall C. A framework to guide the optimal development and use of real-world evidence for drug coverage and formulary decisions. J Comp Eff Res. 2018;7(12):1145–52.
Blommestein HM, Franken MG, Uyl-de Groot CA. A practical guide for using registry data to inform decisions about the cost effectiveness of new cancer drugs: lessons learned from the PHAROS registry. Pharmacoeconomics. 2015;33(6):551–60.
Mohseninejad L, van Gils C, Uyl-de Groot CA, Buskens E, Feenstra T. Evaluation of patient registries supporting reimbursement decisions: the case of oxaliplatin for treatment of stage III colon cancer. Value Health. 2015;18(1):84–90.
Fahr P, Buchanan J, Wordsworth S. A review of the challenges of using biomedical big data for economic evaluations of precision medicine. Appl Health Econ Health Policy. 2019;17(4):443–52.
Sherman RE, Anderson SA, Dal Pan GJ, Gray GW, Gross T, Hunter NL, et al. Real-world evidence: what is it and what can it tell us? N Engl J Med. 2016;375(23):2293–7.
Aggarwal A, Fojo T, Chamberlain C, Davis C, Sullivan R. Do patient access schemes for high-cost cancer drugs deliver value to society? Lessons from the NHS Cancer Drugs Fund. Ann Oncol. 2017;28(8):1738–50.
NHS England. Appraisal and funding of cancer drugs from July 2016 (including the new Cancer Drugs Fund): a new deal for patients, taxpayers and industry. 2016. https://www.england.nhs.uk/wp-content/uploads/2013/04/cdf-sop.pdf. Accessed 07 Oct 2019.
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline E9. 1998. http://academy.gmp-compliance.org/guidemgr/files/E9_GUIDELINE.PDF. Accessed 07 Oct 2019.
KCE Report 283. Horizon scanning for pharmaceuticals: proposal for the Beneluxa Collaboration. http://www.beneluxa.org/sites/beneluxa.org/files/2017-07/Horizon%20scanning_ScientificReport_full.pdf. Accessed 07 Oct 2019.
O’Mahony JF. Beneluxa: what are the prospects for collective bargaining on pharmaceutical prices given diverse health technology assessment processes? Pharmacoeconomics. 2019;37(5):627–30.
Vella Bonanno P, Bucsics A, Simoens S, Martin AP, Oortwijn W, Gulbinovic J, et al. Proposal for a regulation on health technology assessment in Europe: opinions of policy makers, payers and academics from the field of HTA. Expert Rev Pharmacoecon Outcomes Res. 2019;19(3):251–61.
WHO Europe. Medicines reimbursement policies in Europe. http://www.euro.who.int/__data/assets/pdf_file/0011/376625/pharmaceutical-reimbursement-eng.pdf?ua=1. Accessed 07 Oct 2019.
Author information
Authors and Affiliations
Contributions
Caridad Pontes, Corinne Zara, Josep Torrent-Farnell and Merce Obach developed the initial concept and undertook the initial draft. All authors contributed to subsequent drafts based on their experience. All authors approved the submitted paper.
Corresponding author
Ethics declarations
Funding
No funding was received for the conduct of this study or the preparation of this article.
Conflict of interest
Anumber of the co-authors are employed by health authorities or are advisers to them. Caridad Pontes, Corinne Zara, Josep Torrent-Farnell, Merce Obach, Cristina Nadal, Patricia Vella-Bonanno, Michael Ermisch, Renata Curi Hauegen, Jolanta Gulbinovic, Angela Timoney, Antony P. Martin, Tanja Mueller, Anna Nachtnebel, Stephen Campbell, Gisbert Selke, Tomasz Bochenek, Celia C. Rothe, Ileana Mardare, Marion Bennie, Jurij Fürst, Rickard E. Malmstrom and Brian Godman have no conflicts of interest that are directly relevant to the content of this article. Steven Simoens was a speaker at the European Digestive Oncology Research Forum funded by Lilly and participated in an advisory board on the sustainability of cancer care funded by Hexal.
Rights and permissions
About this article
Cite this article
Pontes, C., Zara, C., Torrent-Farnell, J. et al. Time to Review Authorisation and Funding for New Cancer Medicines in Europe? Inferences from the Case of Olaratumab. Appl Health Econ Health Policy 18, 5–16 (2020). https://doi.org/10.1007/s40258-019-00527-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-019-00527-x