Abstract
Purpose of Review
This review provides an up-to-date overview of the evidence relating to how physical inactivity ameliorates cancer-related fatigue. A summary of the postulated biological mechanisms underpinning the relationship is presented.
Recent Findings
Systematic reviews and meta-analyses synthesising the results of randomised controlled trials of physical activity interventions to reduce fatigue broadly conclude that aerobic and combination exercise may be the most helpful, while resistance training alone is less efficacious. Further, light- and moderate-intensity physical activity interventions appeared to reduce fatigue, whereas vigorous-intensity activity may exacerbate the condition. Physical activity interventions result in greater reductions in cancer-related fatigue when delivered post-treatment. Biological mechanisms that may explain how physical activity can improve different elements of cancer-related fatigue include inflammation; the hypothalamic–pituitary–adrenal (HPA) axis and circadian rhythm dysregulation; serotonin dysregulation; and alterations in ATP and muscle metabolism.
Summary
Physical activity is well tolerated by cancer survivors and results in modest improvements in cancer-related fatigue. Much of the research in this field has been from small-scale feasibility trials. In order to help clinicians and allied health professionals tailor exercise prescriptions to individual needs, further research is required. New trials in this field should implement rigorous inclusion criteria, be fully powered to detect effects in sub-group analyses, incorporate multiple sites, and have well-defined control conditions. There is also a need to better understand how physical activity affects different subtypes of cancer-related fatigue.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11(10):597–609. Narrative review summarising the contemporary research on the biological mechanisms, risk factors, and treatment for cancer-related fatigue.
Singer SKS, Zwerenz R, Eckert K, Hofmeister D, Dietz A, Giesinger J, et al. Age- and sex-standardised prevalence rates of fatigue in a large hospital-based sample of cancer patients. Br J Cancer. 2011;3(105):445–51.
Berger AM, Mooney K, Alvarez-Perez A, Breitbart WS, Carpenter KM, Cella D, et al. Cancer-related fatigue, version 2.2015. J Natl Compr Cancer Netw. 2015;13(8):1012–39.
• van Vulpen JK, Peeters PH, Velthuis MJ, van der Wall E, May AM. Effects of physical exercise during adjuvant breast cancer treatment on physical and psychosocial dimensions of cancer-related fatigue: a meta-analysis. Maturitas. 2016;85:104–11. Systematic review and meta-analysis that examined effects of physical activity interventions on different dimensions of cancer-related fatigue (general fatigue, physical fatigue, affective fatigue, cognitive fatigue, reduced activity, reduced motivation).
Kessels E, Husson O, van der Feltz-Cornelis CM. The effect of exercise on cancer-related fatigue in cancer survivors: a systematic review and meta-analysis. Neuropsychiatr Dis Treat. 2018;14:479–94.
Mustian KM, Alfano CM, Heckler C, Kleckner AS, Kleckner IR, Leach CR, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3(7):961–8.
Brandenbarg D, Korsten J, Berger MY, Berendsen AJ. The effect of physical activity on fatigue among survivors of colorectal cancer: a systematic review and meta-analysis. Support Care Cancer. 2018;26(2):393–403.
Lipsett A, Barrett S, Haruna F, Mustian K, O'Donovan A. The impact of exercise during adjuvant radiotherapy for breast cancer on fatigue and quality of life: a systematic review and meta-analysis. Breast. 2017;32:144–55.
Juvet LK, Thune I, Elvsaas IKO, Fors EA, Lundgren S, Bertheussen G, et al. The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: a meta-analysis. Breast. 2017;33:166–77.
Oberoi S, Robinson PD, Cataudella D, Culos-Reed SN, Davis H, Duong N, et al. Physical activity reduces fatigue in patients with cancer and hematopoietic stem cell transplant recipients: a systematic review and meta-analysis of randomized trials. Crit Rev Oncol Hematol. 2018;122:52–9.
• Kelley GA, Kelley KS. Exercise and cancer-related fatigue in adults: a systematic review of previous systematic reviews with meta-analyses. BMC Cancer. 2017;17(1):693. Umbrella review of previous meta-analyses of effects of exercise interventions on cancer-related fatigue.
Tian L, Lu HJ, Lin L, Hu Y. Effects of aerobic exercise on cancer-related fatigue: a meta-analysis of randomized controlled trials. Support Care Cancer. 2016;24(2):969–83.
Carayol M, Delpierre C, Bernard P, Ninot G. Population-, intervention- and methodology-related characteristics of clinical trials impact exercise efficacy during adjuvant therapy for breast cancer: a meta-regression analysis. Psycho-Oncology. 2015;24(7):737–47.
Meneses-Echavez JF, Gonzalez-Jimenez E, Ramirez-Velez R. Effects of supervised multimodal exercise interventions on cancer-related fatigue: systematic review and meta-analysis of randomized controlled trials. Biomed Res Int. 2015;2015:328636.
Yunfeng G, Weiyang H, Xueyang H, Yilong H, Xin G. Exercise overcome adverse effects among prostate cancer patients receiving androgen deprivation therapy: an update meta-analysis. Medicine. 2017;96(27):e7368.
• Fuller JT, Hartland MC, Maloney LT, Davison K. Therapeutic effects of aerobic and resistance exercises for cancer survivors: a systematic review of meta-analyses of clinical trials. Br J Sports Med. 2018, in press https://doi.org/10.1136/bjsports-2017-098285. Umbrella review of meta-analyses quantifying the effects of aerobic and resistance exercise interventions on health outcomes, including fatigue, in cancer survivors.
Cramer H, Lauche R, Klose P, Dobos G, Langhorst J. A systematic review and meta-analysis of exercise interventions for colorectal cancer patients. Eur J Cancer Care. 2014;23(1):3–14.
Dennett AM, Peiris CL, Shields N, Prendergast LA, Taylor NF. Moderate-intensity exercise reduces fatigue and improves mobility in cancer survivors: a systematic review and meta-regression. J Physiotherapy. 2016;62(2):68–82.
Lin KY, Frawley HC, Denehy L, Feil D, Granger CL. Exercise interventions for patients with gynaecological cancer: a systematic review and meta-analysis. Physiotherapy. 2016;102(4):309–19.
Strasser B, Steindorf K, Wiskemann J, Ulrich CM. Impact of resistance training in cancer survivors: a meta-analysis. Med Sci Sports Exerc. 2013;45(11):2080–90.
Zou LY, Yang L, He XL, Sun M, Xu JJ. Effects of aerobic exercise on cancer-related fatigue in breast cancer patients receiving chemotherapy: a meta-analysis. Tumour Biol. 2014;35(6):5659–67.
Higgins J, Green S. Cochrane handbook for systematic reviews of interventions The Cochrane Collaboration; 2011.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713–21.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Meneses-Echavez JF, Gonzalez-Jimenez E, Ramirez-Velez R. Supervised exercise reduces cancer-related fatigue: a systematic review. J Phys. 2015;61(1):3–9.
Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA Caner J Clin. 2012;62(4):243–74.
Wolin KY, Schwartz AL, Matthews CE, Courneya KS, Schmitz KH. Implementing the exercise guidelines for cancer survivors. J Support Oncol. 2012;10(5):171–7.
Duong N, Davis H, Robinson PD, Oberoi S, Cataudella D, Culos-Reed SN, et al. Mind and body practices for fatigue reduction in patients with cancer and hematopoietic stem cell transplant recipients: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;120:210–6.
Thong M, Mols F, van de Poll-Franse L, Sprangers MA, van der Rijt C, Barsevick A, et al. Identifying the subtypes of cancer-related fatigue: results from the population-based PROFILES registry. J Cancer Survivorship. 2018;12:38–46.
Ebede CC, Jang Y, Escalante CP. Cancer-related fatigue in cancer survivorship. Med Clin N Am. 2017;101(6):1085–97.
Vineis P, Perera F. Molecular epidemiology and biomarkers in etiologic cancer research: the new in light of the old. Cancer Epidemiol Biomark Prev. 2007;16(10):1954–65.
Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26(6):971–82.
Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8(11):887–99.
Bower JE, Ganz PA, Tao ML, Hu W, Belin TR, Sepah S, et al. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res. 2009;15(17):5534–40.
Liu L, Mills PJ, Rissling M, Fiorentino L, Natarajan L, Dimsdale JE, et al. Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain Behav Immun. 2012;26(5):706–13.
Wang XS, Shi Q, Williams LA, Mao L, Cleeland CS, Komaki RR, et al. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behav Immun. 2010;24(6):968–74.
Wang XS, Williams LA, Krishnan S, Liao Z, Liu P, Mao L, et al. Serum sTNF-R1, IL-6, and the development of fatigue in patients with gastrointestinal cancer undergoing chemoradiation therapy. Brain Behav Immun. 2012;26(5):699–705.
Alexander S, Minton O, Andrews P, Stone P. A comparison of the characteristics of disease-free breast cancer survivors with or without cancer-related fatigue syndrome. Eur J Cancer. 2009;45(3):384–92.
Alfano CM, Imayama I, Neuhouser ML, Kiecolt-Glaser JK, Smith AW, Meeske K, et al. Fatigue, inflammation, and omega-3 and omega-6 fatty acid intake among breast cancer survivors. J Clin Oncol. 2012;30(12):1280–7.
Orre IJ, Reinertsen KV, Aukrust P, Dahl AA, Fossa SD, Ueland T, et al. Higher levels of fatigue are associated with higher CRP levels in disease-free breast cancer survivors. J Psychosom Res. 2011;71(3):136–41.
Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64(4):604–11.
Bower JE, Ganz PA, Aziz N, Olmstead R, Irwin MR, Cole SW. Inflammatory responses to psychological stress in fatigued breast cancer survivors: relationship to glucocorticoids. Brain Behav Immun. 2007;21(3):251–8.
Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12(9):2759–66.
Orre IJ, Murison R, Dahl AA, Ueland T, Aukrust P, Fossa SD. Levels of circulating interleukin-1 receptor antagonist and C-reactive protein in long-term survivors of testicular cancer with chronic cancer-related fatigue. Brain Behav Immun. 2009;23(6):868–74.
Schrepf A, Clevenger L, Christensen D, DeGeest K, Bender D, Ahmed A, et al. Cortisol and inflammatory processes in ovarian cancer patients following primary treatment: relationships with depression, fatigue, and disability. Brain Behav Immun. 2013;30(Suppl):S126–34.
Bower JE, Ganz PA, Irwin MR, Arevalo JM, Cole SW. Fatigue and gene expression in human leukocytes: increased NF-kappaB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behav Immun. 2011;25(1):147–50.
Lynch BM, Friedenreich CM, Winkler EA, Healy GN, Vallance JK, Eakin EG, et al. Associations of objectively assessed physical activity and sedentary time with biomarkers of breast cancer risk in postmenopausal women: findings from NHANES (2003-2006). Breast Cancer Res Treat. 2011;130(1):183–94.
Yates T, Khunti K, Wilmot EG, Brady E, Webb D, Srinivasan B, et al. Self-reported sitting time and markers of inflammation, insulin resistance, and adiposity. Am J Prev Med. 2012;42(1):1–7.
Meneses-Echavez JF, Correa-Bautista JE, Gonzalez-Jimenez E, Schmidt Rio-Valle J, Elkins MR, Lobelo F, et al. The effect of exercise training on mediators of inflammation in breast cancer survivors: a systematic review with meta-analysis. Cancer Epidemiol Biomark Prev. 2016;25(7):1009–17.
Galvao DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol. 2010;28(2):340–7.
Lee DH, Kim JY, Lee MK, Lee C, Min JH, Jeong DH, et al. Effects of a 12-week home-based exercise program on the level of physical activity, insulin, and cytokines in colorectal cancer survivors: a pilot study. Support Care Cancer. 2013;21(9):2537–45.
Barsevick A, Frost M, Zwinderman A, Hall P, Halyard M. I’m so tired: biological and genetic mechanisms of cancer-related fatigue. Qual Life Res. 2010;19(10):1419–27.
Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR. Mechanisms of cancer-related fatigue. Oncologist. 2007;12(Suppl 1):22–34.
Berger AM, Wielgus K, Hertzog M, Fischer P, Farr L. Patterns of circadian activity rhythms and their relationships with fatigue and anxiety/depression in women treated with breast cancer adjuvant chemotherapy. Support Care Cancer. 2010;18(1):105–14.
Liu L, Rissling M, Neikrug A, Fiorentino L, Natarajan L, Faierman M, et al. Fatigue and circadian activity rhythms in breast cancer patients before and after chemotherapy: a controlled study. Fatigue. 2013;1(1–2):12–26.
Roscoe JA, Morrow GR, Hickok JT, Bushunow P, Matteson S, Rakita D, et al. Temporal interrelationships among fatigue, circadian rhythm and depression in breast cancer patients undergoing chemotherapy treatment. Support Care Cancer. 2002;10(4):329–36.
Mormont M, Waterhouse J. Contribution of the rest-activity circadian rhythm to quality of life in cancer patients. Chronobiol Int. 2002;19(1):313–23.
Tell D, Mathews HL, Janusek LW. Day-to-day dynamics of associations between sleep, napping, fatigue, and the cortisol diurnal rhythm in women diagnosed as having breast cancer. Psychosom Med. 2014;76(7):519–28.
Oster H, Challet E, Ott V, Arvat E, de Kloet ER, Dijk DJ, et al. The functional and clinical significance of the 24-h rhythm of circulating glucocorticoids. Endocr Rev. 2016;38(1):3–45.
Bower JE, Ganz PA, Dickerson SS, Petersen L, Aziz N, Fahey JL. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology. 2005;30(1):92–100.
Schmidt ME, Semik J, Habermann N, Wiskemann J, Ulrich CM, Steindorf K. Cancer-related fatigue shows a stable association with diurnal cortisol dysregulation in breast cancer patients. Brain Behav Immun. 2016;52:98–105.
Weinrib AZ, Sephton SE, Degeest K, Penedo F, Bender D, Zimmerman B, et al. Diurnal cortisol dysregulation, functional disability, and depression in women with ovarian cancer. Cancer. 2010;116(18):4410–9.
Saxton JM, Scott EJ, Daley AJ, Woodroofe M, Mutrie N, Crank H, et al. Effects of an exercise and hypocaloric healthy eating intervention on indices of psychological health status, hypothalamic-pituitary-adrenal axis regulation and immune function after early-stage breast cancer: a randomised controlled trial. Breast Cancer Res. 2014;16(2):R39.
Bailey SP, Davis JM, Ahlborn EN. Neuroendocrine and substrate responses to altered brain 5-HT activity during prolonged exercise to fatigue. J Appl Physiol. 1993;74(6):3006–12.
Kilgour R, Vigano A, Trutschnigg B, Hornby L, Lucar E, Bacon S, et al. Cancer-related fatigue: the impact of skeletal muscle mass and strength in patients with advanced cancer. J Cachexia Sarcopenia Muscle. 2010;1(2):177–85.
Kisiel-Sajewicz K, Davis MP, Siemionow V, Seyidova-Khoshknabi D, Wyant A, Walsh D, et al. Lack of muscle contractile property changes at the time of perceived physical exhaustion suggests central mechanisms contributing to early motor task failure in patients with cancer-related fatigue. J Pain Symptom Manag. 2012;44(3):351–61.
Yavuzsen T, Davis MP, Ranganathan VK, Walsh D, Siemionow V, Kirkova J, et al. Cancer-related fatigue: central or peripheral? J Pain Symptom Manag. 2009;38(4):587–96.
Sorensen JC, Cheregi BD, Timpani CA, Nurgali K, Hayes A, Rybalka E. Mitochondria: inadvertent targets in chemotherapy-induced skeletal muscle toxicity and wasting? Cancer Chemother Pharmacol. 2016;78(4):673–83.
Agteresch H, Dagnelie P, van der Gaast A, Stijnen T, Wilson J. Randomized clinical trial of adenosine 5′-triphosphate in patients with advanced non-small-cell lung cancer. J Natl Cancer Inst. 2000;92(4):321–8.
Carson J, Hardee J, VanderVeen B. The emerging role of skeletal muscle oxidative metabolism as a biological target and cellular regulator of cancer-induced muscle wasting. Semin Cell Dev Biol. 2016;54:53–67.
Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11(9):625–32.
Hansen J, Timmers S, Moonen-Kornips E, Duez H, Staels B, Hesselink MK, et al. Synchronized human skeletal myotubes of lean, obese and type 2 diabetic patients maintain circadian oscillation of clock genes. Sci Rep. 2016;6:35047.
van Moorsel D, Hansen J, Havekes B, Scheer FA, Jorgensen JA, Hoeks J, et al. Demonstration of a day-night rhythm in human skeletal muscle oxidative capacity. Mol Metab. 2016;5(8):635–45.
Haberlin C, O'Dwyer T, Mockler D, Moran J, O'Donnell DM, Broderick J. The use of eHealth to promote physical activity in cancer survivors: a systematic review. Support Care Cancer. 2018;
Phillips SM, Cadmus-Bertram L, Rosenberg D, Buman MP, Lynch BM. Wearable technology and physical activity in chronic disease: opportunities and challenges. Am J Prev Med. 2018;54(1):144–50.
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 Lyon, France: International Agency for Research on Cancer 2013.
Funding
van Roekel was supported by an Endeavour Research Fellowship from the Department of Education and Training of the Australian Government (6059-2017); Lynch was funded by a National Breast Cancer Foundation Fellowship (ECF-15-012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Bernat-Carles Serdà i Ferrer, Eline van Roekel, and Brigid M. Lynch declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Cancer
Appendix
Appendix
Aim
Search for systematic reviews and meta-analyses focused on physical activity (exposure or intervention) and cancer-related fatigue (outcome) in adults diagnosed with any stage of cancer, at any time post-diagnosis (including palliative care).
Data Sources and Search Strategy
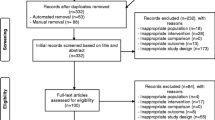
The search was focused on systematic reviews and meta-analyses published from January 2013 to May of 2018 and updated to Jun 2018. The literature search was performed using MEDLINE and PsycINFO. All searches were conducted by one author [BCSF]. All references were imported into EndNote X5.
Eligibility Criteria
Inclusion criteria:
-
Fatigue as a main outcome.
-
Physical activity intervention.
-
Primary participants had a diagnosis of cancer.
-
Reviews and/or meta-analysis.
-
Studies written in Spanish, English, or French.
Exclusion criteria:
-
Other chronic diseases.
-
Not using physical activity as primary intervention.
PubMed Search
The search strategy for identification of the studies included Medical Subject Heading terms combined with the Boolean terms as follows:
‘Exercise’ [Mesh] OR ‘Exercise Therapy’ [Mesh] OR ‘Resistance Training’ [Mesh] OR ‘Sports’ [Mesh] OR ‘Yoga’ [Mesh] AND ‘Neoplasms’ [Mesh] AND ‘Fatigue’ [Mesh]
Filters: Meta-Analysis, Review; From 01/01/2013 to 12/06/2018. The search identified 58 potentially relevant reviews.
PsycINFO
The search strategy implemented were ‘Cancer’ ‘Exercise’ OR ‘Physical activity’ AND ‘Cancer-related Fatigue’ AND Methodology Systematic Review OR Meta Analysis AND Peer-Reviewed Journals Only AND Year: 2013 to 2018 and identified 90 potentially relevant reviews.
Rights and permissions
About this article
Cite this article
Serdà i Ferrer, BC., van Roekel, E. & Lynch, B.M. The Role of Physical Activity in Managing Fatigue in Cancer Survivors. Curr Nutr Rep 7, 59–69 (2018). https://doi.org/10.1007/s13668-018-0234-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13668-018-0234-1