Abstract
Background
Succinylcholine provides rapid onset of neuromuscular blockade and short duration of action, but its administration may be associated with hyperkalemia. Rocuronium is not known to increase potassium concentration, has fast onset of activity, and can be rapidly reversed by sugammadex. This study evaluated changes in plasma potassium concentrations in patients randomized either to rocuronium followed by sugammadex reversal or to succinylcholine in ambulatory surgery.
Methods
In this multicentre randomized active-controlled study, adult patients undergoing short surgical procedures in an outpatient setting received either rocuronium 0.6 mg·kg−1 for intubation with sugammadex 4.0 mg·kg−1 for reversal (n = 70) or succinylcholine 1.0 mg·kg−1 with spontaneous recovery (n = 80). Blood potassium concentrations were assessed at baseline (before study drug administration) and at intervals up to 15 min after rocuronium, sugammadex, and succinylcholine.
Results
At the primary endpoint, five minutes post-administration, the changes in potassium concentrations from baseline were significantly smaller in patients treated with rocuronium than in those given succinylcholine [mean (SD): −0.06 (0.32) vs 0.30 (0.34) mmol·L−1, respectively; P < 0.0001]. At baseline, potassium concentrations were similar in both groups, but they were greater at two, five, ten, and 15 min after succinylcholine than after rocuronium (P < 0.0001) for all time points. After sugammadex administration, there were no significant changes in mean potassium concentration from the pre-rocuronium baseline. No adverse effects related to hyperkalemia were observed.
Conclusion
Succinylcholine was associated with a modest increase in potassium concentration; these changes were not seen after rocuronium or sugammadex (Clinical trial registration number: NCT00751179).
Résumé
Contexte
La succinylcholine entraîne l’installation rapide d’un blocage neuromusculaire avec une courte durée d’action, mais son administration peut être associée à une hyperkaliémie. Le rocuronium n’est pas réputé pour augmenter la concentration du potassium, a un court délai d’apparition de l’activité et son activité peut être rapidement annulée par le sugammadex. Cette étude a évalué les variations de la concentration plasmatique de potassium chez des patients randomisés ayant reçu du rocuronium suivi d’une annulation par sugammadex, ou de la succinylcholine en chirurgie d’un jour.
Méthodes
Dans cette étude randomisée, multicentrique, contrôlée contre produit actif, des patients adultes subissant de brèves interventions chirurgicales dans un cadre de chirurgie d’un jour ont reçu du rocuronium 0,6 mg·kg−1 pour l’intubation puis du sugammadex 4,0 mg·kg−1 pour en contrer l’effet (n = 70) ou de la succinylcholine 1,0 mg·kg−1 avec une récupération spontanée (n = 80). La concentration du potassium sanguin a été évaluée à la ligne de base (avant l’administration du médicament étudié), puis à intervalles allant jusqu’à 15 minutes après l’administration de rocuronium, sugammadex et succinylcholine.
Résultats
À la ligne de base, les concentrations de potassium étaient comparables dans les deux groupes, mais elles ont été plus élevées à 2, 5, 10 et 15 min après l’administration de succinylcholine, par rapport à l’administration de rocuronium (P < 0,0001 pour tous les points de référence). Cinq minutes après l’administration, les modifications de la concentration de potassium par rapport à la ligne de base étaient significativement plus faibles chez les patients ayant reçu du rocuronium que chez les patients ayant reçu de la succinylcholine (moyenne [E.-T.]: respectivement −0,06 [0,32] contre 0,30 [0,34] mmol·L−1; P < 0,0001]. Après l’administration de sugammadex, il n’y a pas eu de modification significative de la concentration moyenne du potassium par rapport à la valeur de référence pré-rocuronium. Aucun effet secondaire lié à hyperkaliémie n’a été observé.
Conclusion
La succinylcholine a été associée à une modeste augmentation de la concentration du potassium; ces changements n’ont pas été observés après l’administration de rocuronium ou de sugammadex (Numéro d’enregistrement de l’essai clinique: NCT00751179).
Similar content being viewed by others
Due to its rapid onset and short duration of action, succinylcholine may be used in outpatient surgery to facilitate tracheal intubation.1 Nevertheless, succinylcholine is associated with an increase in blood potassium concentrations due to its mechanism of action (i.e., depolarization of the muscle cell membrane by the opening of cholinergic receptors, which results in the release of potassium into the intracellular space).2 In extreme cases, hyperkalemia (defined as a plasma potassium concentration > 5.5 mmol·L−1) may be associated with serious arrhythmias, including ventricular fibrillation and asystolic arrest.3-5 Other adverse effects of succinylcholine can include bradyarrhythmias, fasciculations, and myalgia, as well as malignant hyperthermia in susceptible patients.1,6 , Footnote 1
Anesthesiologists may instead consider using a non-depolarizing neuromuscular blocking agent (NMBA). A disadvantage of this practice, however, is that the patient may have unintended residual blockade at the end of surgery, which may lead to aspiration, hypoxia, muscle weakness, and respiratory complications.7-9 Residual blockade is a particular concern in brief outpatient procedures. Rocuronium has a rapid onset of activity, but its duration of action may be too long for many outpatient procedures. Then again, the availability of sugammadex to provide predictable, complete, and rapid reversal of rocuronium-induced neuromuscular blockade (NMB)10,11 may allow the use of rocuronium to facilitate tracheal intubation in the outpatient setting as well as permit continued relaxation up until the end of surgery. Furthermore, this combination of agents has less potential for increases in potassium concentration compared with succinylcholine, as rocuronium blocks acetylcholine receptor channels competitively, and its mechanism of action is not associated with potassium release.
The primary objective of this study was to evaluate changes in plasma potassium concentrations in patients randomized to treatment with rocuronium followed by sugammadex reversal vs succinylcholine in patients scheduled for short ambulatory surgical procedures.
Methods
This was a multicentre randomized safety-assessor blinded parallel-group active-controlled trial in adult patients scheduled for short surgical procedures in outpatient surgical centres. The study was conducted in accordance with principles of good clinical practice and was approved by the appropriate institutional review boards and regulatory agencies (Table 1). All patients provided written informed consent before enrolment.
Patients ≥ 18 yr of age were eligible for inclusion if they were American Society of Anesthesiologists physical status class I-III, had a body mass index of < 35 kg·m−2, and were scheduled to undergo an elective outpatient procedure expected to last ≤ 1.5 hr (from completion of intubation to completion of suturing/stapling of skin) under general anesthesia requiring neuromuscular relaxation for tracheal intubation. Neuromuscular monitoring was performed using the TOF-Watch® SX device (Organon Ireland Ltd., a subsidiary of Merck and Co., Swords, Co. Dublin, Ireland) affixed to an outstretched arm, and each study site was given training on the set-up and calibration of the TOF-Watch® SX to reduce variability between sites. Exclusion criteria included patients: with ischemic heart disease or a history of myocardial infarction; expected to have a difficult tracheal intubation due to anatomical malformations; with neuromuscular disorders potentially altering responses to NMB (e.g., myasthenia gravis); requiring the use of a pneumatic tourniquet during the surgical procedure; with significant renal (creatinine clearance < 30 mL·min−1) or hepatic dysfunction; with a family history of malignant hyperthermia; with an allergy to opioids, muscle relaxants, or other medications used during general anesthesia; hypersensitive to sugammadex, other cyclodextrins, or rocuronium bromide; with a contraindication to succinylcholine, rocuronium, or sugammadex; with a pre-established need for postoperative intensive care admission and/or an anticipated hospital admission; and expected to receive an intraoperative intravenous administration of fluids containing potassium. Patients who participated in a previous sugammadex trial or in another investigational drug trial and female patients who were pregnant or breastfeeding were also excluded.
Treatments
Patients were randomized (1:1) using a secured-trial web-based randomization system to receive either rocuronium and sugammadex or succinylcholine for NMB. Patients in the rocuronium-sugammadex group received rocuronium 0.6 mg·kg−1 as a single intravenous bolus dose for tracheal intubation and then rocuronium 0.15 mg·kg−1 for maintenance of NMB as required. This was followed by sugammadex 4.0 mg·kg−1 administered intravenously for NMB reversal at a target depth of NMB of one to two post-tetanic counts following the last dose of rocuronium. In the other group, succinylcholine 1.0 mg·kg−1 was administered as a single intravenous bolus dose for tracheal intubation. Patients in this group were allowed to recover spontaneously with no subsequent NMBA administration.
Anesthesia was induced with propofol, intravenous opioids, and other medications or agents and was maintained with volatile anesthetics, intravenous opioids, propofol, or other medications or agents as required. Following induction of anesthesia and prior to administration of rocuronium or succinylcholine, the TOF-Watch® SX was affixed, stabilized, and calibrated to monitor neuromuscular function continuously at the adductor pollicis muscle until the train-of-four (TOF) ratio was ≥ 0.9 (in the rocuronium-sugammadex group) or first twitch (T1) was ≥ 90% of baseline (in the succinylcholine group).
Evaluations
The changes in plasma potassium concentrations from baseline were evaluated following induction of NMB with rocuronium or succinylcholine and again following administration of sugammadex to reverse rocuronium-induced NMB.
Blood samples for the evaluation of potassium concentrations were collected using a tourniquet to draw blood through a dedicated intravenous cannula inserted into the patient’s upper extremity contralateral to the site of the intravenous line used to administer medications. Samples were drawn just prior to administration of rocuronium or succinylcholine (baseline) and at two, five, ten, and 15 min after the start of administration of NMBA. In the rocuronium group, additional blood samples were taken five minutes after each maintenance dose. Blood samples were then collected at two, five, ten, and 15 min after the start of administration of sugammadex. The blood samples were sent to a central laboratory (Bio Analytical Research Corporation [BARC], Lake Success, New York, NY, USA) for analysis of potassium concentrations. Central laboratory staff members were not blinded to the treatment group allocations. Before potassium levels were determined, blood samples were assessed for the presence of hemolysis (mild, moderate, or gross). Blood samples which were determined to have undergone hemolysis (which can lead to artificially high potassium concentrations) were excluded from the primary analysis. If a baseline sample was hemolyzed, all potassium data for that patient were excluded from the analyses.
The normal range for potassium values for the central laboratory was defined as 3.4-4.5 mmol·L−1. Clinically significant, markedly abnormal laboratory values were defined as any potassium value outside the range of 3.06-4.95 mmol·L−1. Pre-specified notable increases in potassium concentration were defined either as values ≥ 4.5 mmol·L−1 in the last measurement after administration of the study drug (endpoint value) when baseline value was < 4.5 mmol·L−1, or as endpoint values ≥ 4.95 mmol·L−1 when baseline value was below the upper limit of safety (4.95 mmol·L−1).
In addition to potassium levels, safety was assessed by reporting adverse events (AEs) as well as by evaluating vital signs (heart rate, blood pressure [assessed using a blood pressure cuff contralateral to the site of the intravenous line used to administer medications], respiratory rate, and pulse oximetry), including identification of markedly abnormal vital sign values, defined as follows:
-
Systolic blood pressure: ≥ 160 mmHg in combination with an increase of ≥ 20 mmHg from baseline or ≤ 90 mmHg in combination with a decrease of ≥ 20 mmHg from baseline;
-
Diastolic blood pressure: ≥ 95 mmHg in combination with an increase of ≥ 15 mmHg from baseline or ≤ 45 mmHg in combination with a decrease of ≥ 15 mmHg from baseline;
-
Heart rate: ≥ 120 beats·min−1 or ≤ 50 beats·min−1 in combination with an increase of ≥ 15 beats·min−1 or a decrease of ≥ 15 beats·min−1 from baseline, respectively.
All AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA Version 12.1, McLean, VA, USA). Furthermore, a number of additional measures of safety were made to detect evidence of postoperative complications/AEs, including assessment of postoperative myalgia, respiratory complications, and serious AEs.
The efficacy endpoint in the rocuronium-sugammadex group was the time from the start of administration of sugammadex to recovery of the TOF ratio to 0.9. To evaluate neuromuscular recovery in the succinylcholine group, the time was assessed from the start of administration of succinylcholine to T1 reaching 90% of baseline.
Populations
The pre-specified all-subjects-treated population (AST) was defined as all randomized patients who received succinylcholine or sugammadex. However, as the pre-specified AST population excluded the patients who received rocuronium but did not subsequently receive sugammadex, changes from baseline in potassium concentrations were determined in a post-hoc AST population which included all patients who received any study drug.
Statistical analyses
The sample-size calculations were based on the previously reported mean (SD) increase of 0.36 (0.23) mmol·L−1 in potassium concentrations from baseline to five minutes post-succinylcholine administration.12 A reduction of 50% in the increase in potassium concentration after rocuronium or sugammadex compared with the increase observed after succinylcholine was considered a meaningful reduction. From this, the required sample size was calculated to be 54 patients per group. To allow for dropouts after the initial treatment period and to provide sufficient power to address the primary and secondary outcomes, 70 patients per group were to be enrolled.
The changes in plasma potassium concentration from baseline (pre-intubation values prior to rocuronium or succinylcholine administration) were calculated for samples obtained at two, five, ten, and 15 min (when available) after administration of doses of rocuronium or succinylcholine for tracheal intubation and after sugammadex. Potassium levels were also measured for samples obtained at five minutes after each maintenance dose of rocuronium; however, potassium levels obtained after maintenance doses were not included in the primary analysis. Summary statistics of potassium levels were determined for each post-rocuronium intubation dose, post-sugammadex dose, and post-succinylcholine dose by treatment group. Summary statistics of the change in potassium level from baseline were calculated for each treatment group.
A comparison of the change in potassium concentrations from baseline was made between rocuronium and succinylcholine treatment using a one-way analysis of variance model with treatment as the term in the model and a significance level of 0.05. The change in potassium level from baseline at five minutes after study drug administration was considered to be of particular interest and was therefore defined as the primary outcome measurement. Potassium levels obtained at the other time points were to be treated as secondary endpoints. In addition, the change in potassium values from baseline was tested within each treatment group using a paired Student’s t test. For these secondary and other endpoints, no adjustments were made for multiple comparisons.
Results
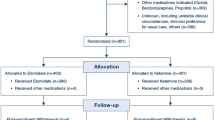
The study was carried out from December 2008 to December 2009 at 13 sites in the USA and one in Canada (Table 1). One hundred sixty-one of the 167 patients enrolled in the study were randomized to treatment (Fig. 1); randomization ran from 8 December 2008 to 23 November 2009. Six enrolled patients were not randomized and did not participate in the study due to: withdrawal of informed consent (n = 3), failure to meet protocol eligibility criteria (n = 1), a technical problem with the web-based randomization system (n = 1), and withdrawal due to death in the investigator’s family (n = 1).
Patient flow chart. aOne patient received succinylcholine but tracheal intubation failed. Discontinuation was reported as a pre-treatment non-serious adverse event, and this patient was erroneously included in the all-subjects-treated and intent-to-treat (received study drug and had at least one efficacy measure) populations. Nevertheless, this patient had no further safety or efficacy measures and therefore contributed no additional information to these populations
One hundred fifty patients from the 161 randomized patients received the assigned treatment (AST population), 70 patients in the rocuronium-sugammadex group and 80 patients in the succinylcholine group. Six patients randomized to rocuronium-sugammadex did not receive treatment due to: withdrawal of informed consent (n = 2); patient not fulfilling inclusion/exclusion criteria (n = 1); inability to calibrate the TOF-Watch® SX (n = 1); unstable TOF-Watch® SX set-up resulting in an unacceptable delay to surgery (n = 1); and cancellation of surgery (n = 1). Five patients randomized to succinylcholine did not receive the assigned study treatment due to: patient not fulfilling inclusion/exclusion criteria (n = 1); inability to calibrate the TOF-Watch® SX (n = 2); TOF-Watch® SX failure due to the electrodes being past the expiration date (n = 1); and non-availability of succinylcholine (n = 1).
Patient characteristics were comparable between the rocuronium-sugammadex and succinylcholine groups (Table 2). Patients most frequently underwent surgical procedures involving gynecological surgery, eye and adjacent structures, and the digestive system and spleen.
Changes in potassium concentrations during treatment
In the rocuronium-sugammadex group, blood samples from three patients were not available for assessment of potassium concentration, baseline samples from five patients were hemolyzed, and baseline samples from a further three patients were missing. Thus, samples from 59 patients were analyzed for potassium concentrations. Blood samples from one patient in the succinylcholine group were not available, and baseline samples from seven patients were hemolyzed. Thus, samples from 72 subjects were analyzed for potassium concentrations in the succinylcholine group.
Baseline potassium concentrations were similar between the treatment groups (Table 3). Following administration of rocuronium, mean potassium concentration decreased slightly at two minutes (P < 0.05) but then returned to baseline values (Table 3, Fig. 2). Following administration of succinylcholine, mean potassium concentration increased by approximately 0.2 mmol·L−1 at two minutes with an additional small increase at five minutes. This was followed by little further change up to 15 min post dose (all P < 0.0001 vs baseline) (Table 3, Fig. 2). The mean (SD) change in potassium levels from baseline at five minutes (primary outcome measurement) was -0.06 (0.32) mmol·L−1 in patients treated with rocuronium compared with +0.30 (0.34) mmol·L−1 in patients treated with succinylcholine (mean difference 0.36 mmol·L−1; 95% confidence intervals 0.24 to 0.47 mmol·L−1; P < 0.0001). Potassium concentrations after administration of rocuronium were also significantly smaller (P < 0.0001) than after succinylcholine at all other time points up to 15 min post dose (Table 3).
The box plot illustrates change in plasma potassium concentration (mmol·L−1) from baseline following rocuronium, sugammadex, and succinylcholine, respectively. Represented are the mean (+), median (horizontal line) and the interquartile range (IQR) (box). The 25th and 75th percentiles are at the lower and upper box edges, respectively. The extreme values that are more than 1.5 times the IQR above the 75th percentile or below the 25th percentile are marked individually, and the whisker is drawn at the most extreme of the remaining values. Values are presented at two, five, ten, and 15 min post-administration. *P < 0.05; **P < 0.0001
Following administration of sugammadex, mean plasma potassium concentrations were similar to the pre-rocuronium baseline at two and five minutes, with a maximum mean (SD) difference from baseline of 0.02 (0.42) mmol·L−1 (Fig. 2). There was a slight (0.07 mmol·L−1) increase from baseline in mean potassium concentration at 10 min, which was sustained at 15 min; these slight differences were not significant and were not determined to be clinically meaningful (Table 3, Fig. 2). Direct comparisons were not made between changes in potassium concentration from baseline with sugammadex and succinylcholine as the time points relative to the beginning of surgery were not comparable; however, while succinylcholine was associated with modest increases in plasma potassium levels, the combination of rocuronium and sugammadex did not result in an increased potassium concentration.
Notable increases in plasma potassium concentrations (defined either as a last potassium concentration measurement ≥ 4.5 mmol·L−1 with a < 4.5 mmol·L−1 baseline or as a last value of ≥ 4.95 mmol·L−1 with a baseline value < 4.95 mmol·L−1) were seen in four patients in the rocuronium-sugammadex treatment group. One patient showed a notable increase at 15 min after rocuronium, and this level was then maintained throughout the study until 15 min after sugammadex administration. In three other patients, the notable increase was seen only 15 min after sugammadex administration. After administration of succinylcholine, 13 patients showed a notable increase in plasma potassium concentration. One rocuronium-sugammadex patient and one succinylcholine patient had potassium concentration values ranging from 5.0 to 5.2 mmol·L−1, which were above the upper safety threshold of 4.95 mmol·L−1 at any point during the study. One AE of hyperkalemia was reported from non-hemolyzed blood samples. This occurred in a patient in the rocuronium-sugammadex group who was reported as having a potassium concentration of 5.2 mmol·L−1 at five minutes after administration of a maintenance dose of rocuronium (baseline 4.2 mmol·L−1). The potassium concentrations in this patient remained above 5.0 mmol·L−1 following administration of a second maintenance dose of rocuronium and following administration of sugammadex up to 15 min.
On the other hand, notable decreases in potassium concentrations (defined either as a last potassium concentration measurement ≤ 3.06 mmol·L−1 from a baseline value > 3.06 mmol·L−1 or as a last value ≤ 3.4 mmol·L−1 from a baseline value > 3.4 mmol·L−1) were observed in six patients after rocuronium, six patients after sugammadex, and three patients after succinylcholine. No safety concerns were raised by these decreases in potassium. The lowest observed values from baseline to the last potassium measurement were 1.7 mmol·L−1 after the intubating rocuronium dose, 1.8 mmol·L−1 after sugammadex, and 2.0 mmol·L−1 after succinylcholine.
Other safety assessments
Treatment with rocuronium-sugammadex or succinylcholine was generally well tolerated in this outpatient population, with the most frequently occurring AEs shown in Table 4. Sixty-one (87.1%) of the 70 patients in the rocuronium-sugammadex group experienced AEs, and 75 (93.8%) of the 80 patients in the succinylcholine group experienced AEs. One patient who received rocuronium plus sugammadex experienced a serious AE of procedural nausea which was considered unrelated to the study medication. Three patients in the succinylcholine group experienced one serious AE each (rectal hemorrhage, medical observation, and decreased oxygen saturation). All serious AEs were considered unrelated to the study medication. Myalgia was reported for seven (8.8%) patients in the succinylcholine treatment group and for one (1.4%) patient in the rocuronium plus sugammadex treatment group.
Subjects in the succinylcholine group had a higher incidence (21.3%) of procedural hypotension than patients in the rocuronium-sugammadex group (5.7%) (P = 0.008; Table 4). Nevertheless, the overall mean changes in blood pressure were similar between the groups, and the magnitude of changes was generally small and consistent with that typically seen in the perioperative setting.
Efficacy
The mean (SD) recovery time to a TOF ratio ≥ 0.9 after administration of sugammadex in the intent-to-treat population was 2.0 (1.1) min (n = 59). In the succinylcholine group, the mean (SD) time to recovery of T1 to 90% following the administration of succinylcholine was 11.2 (2.9) min (n = 77).
Discussion
This study evaluated the changes in plasma potassium concentrations after administration of rocuronium 0.6 mg·kg−1 with maintenance doses of 0.15 mg·kg−1 as required, followed by reversal with sugammadex 4.0 mg·kg−1. These results were compared with changes after administration of succinylcholine 1.0 mg·kg−1 followed by spontaneous reversal. Following administration of rocuronium, potassium concentrations decreased slightly at two minutes, and from then on, plasma potassium levels were generally unchanged from baseline. By comparison, plasma potassium concentrations increased from baseline at every time point analyzed following administration of succinylcholine. The increases in potassium concentration in the succinylcholine group were significantly greater than those observed in the rocuronium group at every time point from two to 15 min after administration.
In general, mean changes in potassium concentration from baseline following administration of sugammadex after rocuronium were slight and considered not to be clinically meaningful. It should be emphasized, however, that any changes in plasma potassium levels from baseline following sugammadex are more difficult to interpret, since the baseline level was obtained prior to intubation and surgery, and sugammadex was not administered to reverse NMB until after the surgical procedure. At this latter time, assessment of plasma potassium relative to baseline could have been confounded by a number of factors, including the intervening surgical procedure, administration of intravenous fluids, and administration of perioperative medications (including rocuronium). While the lack of a separate baseline prior to the administration of sugammadex may be perceived as a limitation of the current study, the main focus was to compare the combination of rocuronium/sugammadex vs succinylcholine rather than to look at the effect of sugammadex alone. Furthermore, in clinical practice, sugammadex would not be administered without prior administration of a NMBA.
For these reasons, a direct comparison of changes in potassium concentration following sugammadex and succinylcholine would not be appropriate because of the different points of administration during the surgical procedure. Fewer patients in the rocuronium-sugammadex treatment group showed a notable increase in potassium values from baseline to last measurement after administration of the study drug compared with patients in the succinylcholine group (four vs 13 patients, respectively), but this difference was not statistically significant. During the study, potassium values above the upper safety threshold were reported in three patients in the rocuronium-sugammadex group and one patient in the succinylcholine treatment group. Nevertheless, the results of this study suggest that succinylcholine is associated with a greater increase in potassium concentrations overall than the combination of rocuronium for induction and maintenance of NMB followed by sugammadex reversal.
In the current study, the mean increase observed with succinylcholine (0.3 mmol·L-1) at five minutes after dosing is similar to previously reported mean increases in plasma potassium of 0.3-0.54 mmol·L−1 determined five minutes after succinylcholine administration.12 Although the observed changes in potassium after succinylcholine were very modest on average in these studies, patients with preexisting unexpectedly high potassium concentrations may still be at risk of hyperkalemia. Furthermore, with the shift towards more medically complicated patients receiving surgery in the outpatient setting and a decrease in routine preoperative potassium screening, patients with undiagnosed abnormally high potassium concentrations may present for anesthesia and surgery.
Additionally, there were some instances in both groups of a notable decrease in potassium from baseline after administration of the study drug (six patients after rocuronium, six after sugammadex, and three after succinylcholine). The reason for the decline in individual patients was not identified but may reflect changes in acid-base status in response to changes in ventilatory patterns, fluids administered, volume status, or other factors that were not directly controlled or measured as part of the protocol. These changes were judged not to be of clinical concern.
Efficacy results were as expected, with sugammadex 4 mg·kg−1 providing rapid recovery from rocuronium-induced NMB to a TOF ratio of 0.9 in a mean of 2.0 min. The mean time to recovery of T1 to 90% following succinylcholine was 11.2 min. All the study drugs were generally well tolerated in this study, with only one serious AE reported in the rocuronium-sugammadex group and three reported in the succinylcholine group. In the current study, seven patients (8.8%) reported myalgia after succinylcholine, as assessed by AE reporting, compared with one patient (1.4%) in the rocuronium plus sugammadex group. Fasciculations were not specifically reported since they are an expected side effect of succinylcholine and thus not recorded as an AE.
The results of this study suggest that administration of rocuronium for intubation and maintenance of NMB, followed by reversal with sugammadex, is associated with significantly smaller increases in potassium concentrations compared with administration of succinylcholine in outpatient surgery.
Notes
Anectine (suxamethonium chloride). Summary of Product Characteristics, January 2009. Available from URL: http://www.medicines.org.uk/EMC/medicine/704/SPC/Anectine+Injection/ (accessed January 2014).
References
Bettelli G. Which muscle relaxants should be used in day surgery and when. Curr Opin Anaesthesiol 2006; 19: 600-5.
Martyn JA, Richtsfeld M. Succinylcholine-induced hyperkalemia in acquired pathologic states: etiologic factors and molecular mechanisms. Anesthesiology 2006; 104: 158-69.
Gronert GA. Cardiac arrest after succinylcholine: mortality greater with rhabdomyolysis than receptor upregulation. Anesthesiology 2001; 94: 523-9.
Piotrowski AJ, Fendler WM. Hyperkalemia and cardiac arrest following succinylcholine administration in a 16-year-old boy with acute nonlymphoblastic leukemia and sepsis. Pediatr Crit Care Med 2007; 8: 183-5.
Taylor B, MacEwen P. Asystole after alfentanil-succinylcholine. Can J Anaesth 1989; 36: 255-6.
Booij LH. Is succinylcholine appropriate or obsolete in the intensive care unit? Crit Care 2001; 5: 245-6.
Murphy GS, Szokol JW, Marymont JH, Greenberg SB, Avram MJ, Vender JS. Residual neuromuscular blockade and critical respiratory events in the postanesthesia care unit. Anesth Analg 2008; 107: 130-7.
Murphy GS, Brull SJ. Residual neuromuscular block: lessons unlearned. Part 1: definitions, incidence, and adverse physiologic effects of residual neuromuscular block. Anesth Analg 2010; 111: 120-8.
Plaud B, Debaene B, Donati F, Marty J. Residual paralysis after emergence from anesthesia. Anesthesiology 2010; 112: 1013-22.
Jones RK, Caldwell JE, Brull SJ, Soto RG. Reversal of profound rocuronium-induced blockade with sugammadex: a randomized comparison with neostigmine. Anesthesiology 2008; 109: 816-24.
Blobner M, Eriksson LI, Scholz J, Motsch J, Della Rocca G, Prins ME. Reversal of rocuronium-induced neuromuscular blockade with sugammadex compared with neostigmine during sevoflurane anaesthesia: results of a randomised, controlled trial. Eur J Anaesthesiol 2010; 27: 874-81.
Raman SK, San WM. Fasciculations, myalgia and biochemical changes following succinylcholine with atracurium and lidocaine pretreatment. Can J Anaesth 1997; 44: 498-502.
Acknowledgements
The authors sincerely thank Professor Talmage Egan (Department of Anesthesiology, University Hospital, UT, USA) for his assistance in the conduct of the study and his review of early drafts of the manuscript and Joris de Bie (Merck Sharp & Dohme Corp., Whitehouse Station, NJ, USA) for assistance with the design and execution of the study. The authors also thank all investigators involved in the conduct of the study. Medical writing support was provided by Caroline Shapland and Neil Venn from Prime Medica (Knutsford, Cheshire, UK) during the preparation of this manuscript, supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co. Inc., Whitehouse Station, NJ, USA. Responsibility for opinions, conclusions, and interpretation of the data lies with the authors.
List of investigators
Scott Beattie, MD (University Health Network [Princess Margaret Hospital], Toronto, Canada); Ian Black, MD (Fletcher Allen Health Care, Colchester, VT); Benoit Boure, MD (Hopital du Sacre-Coeur de Montreal, Montreal, Canada); Enrico Camporesi, MD (Tampa General Hospital, Tampa, FL); Dawn Desiderio, MD (Memorial Sloan Kettering, New York, NY); Joseph Foss, MD (The Cleveland Clinic Foundation, Cleveland, OH); Vincent Odenigbo, MD (Drexel University, Philadelphia, PA); Robert Redfern, MD (Shands Jacksonville Medical Center, Jacksonville, FL); Scott Siegel, MD (St. Peter’s University Hospital, New Brunswick, NJ); Michael Sutherland, MD (Fletcher Allen Health Care, Colchester, VT); Michael Tessler, MD (SMDB-Jewish General Hospital, Montreal, Canada); Nancy Wilkes, MD (University of North Carolina, Chapel Hill, NC).
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was sponsored by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co. Inc., Whitehouse Station, NJ, USA.
Daniel Sabo, Jonathan Jahr, Janet Pavlin, Beverly Philip, and Roy Soto work for institutions that received research funding from Merck for the conduct of this study. At the time of the study, Noriko Shimode worked for an institution that received research funding from Merck for the conduct of this study. Jonathan Jahr has also served as a consultant and as part of a speaker’s bureau for Merck. Jonathan Jahr, Janet Pavlin, and Roy Soto have also received travel support from Merck. Tiffany Woo is an employee of Merck Sharp & Dohme Corp., Whitehouse Station, NJ; Everton Rowe is a former employee of Merck Sharp & Dohme Corp., Whitehouse Station, NJ.
Author contributions
Jonathan Jahr, Beverly Philip, Everton Rowe, Tiffany Woo, and Roy Soto helped design the study. Daniel Sabo, Jonathan Jahr, Janet Pavlin, Beverly Philip, Noriko Shimode, and Roy Soto were involved in the conduct of the study and data collection. All authors participated in data analysis, contributed to development of the first draft of the manuscript via teleconference, and critically reviewed later drafts during manuscript preparation.
Rights and permissions
About this article
Cite this article
Sabo, D., Jahr, J., Pavlin, J. et al. The increases in potassium concentrations are greater with succinylcholine than with rocuronium-sugammadex in outpatient surgery: a randomized, multicentre trial. Can J Anesth/J Can Anesth 61, 423–432 (2014). https://doi.org/10.1007/s12630-014-0128-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-014-0128-7