Abstract
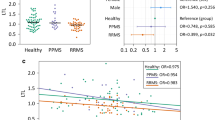
Lipid peroxidation due to oxidative stress (OS) may play an important role in the pathogenesis of chronic systemic inflammatory diseases such as multiple sclerosis (MS). Telomeres, repeated sequences that cap chromosome ends, undergo shortening with each cycle of cell division, resulting in cellular senescence. Research regarding telomere shortening has provided novel insight into the pathogenesis of various diseases. We hypothesized that OS damage leads to inflammatory reactions, which subsequently shortens the telomere length in MS. We enrolled 59 patients with MS, and age- and gender-matched 60 healthy controls. We divided MS subjects into three groups matched for age and gender according to the severity of disability: relatively benign course (BMS), secondary progressive MS, and primary progressive MS (PPMS). We analyzed the telomere length in peripheral blood mononuclear cells and the 8-iso-PGF2α concentration in urine, a reliable and stable marker of lipid peroxidation in vivo. The data showed significant higher levels of urinary 8-iso-PGF2α in MS subjects than in the controls. The lag-time, which represents the direct measurement of the resistance of low-density lipoprotein to oxidation, was shorter in the PPMS subjects than in the groups. Compared to that observed in the controls, the mean telomere length was significantly shorter in the PPMS group, whereas no significant telomere shortening was found between the controls and other subjects. Our data suggest that a decreased telomere length and enhanced lipid peroxidation reflects the severest stage of MS.
Similar content being viewed by others
References
Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG (2000) Multiple sclerosis. N Engl J Med 343:938–952
Zakian VA (1995) Telomeres: beginning to understand the end. Science 270:1601–1607
Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ (2003) White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol 23:842–846
Friedrich U, Gries E, Schwab M, Fritz P, Thon K, Klotz U (2000) Telomere length in different tissues of elderly patients. Mech Ageing 119:89–99
Gilgun-Sherki Y, Melamed E, Offen D (2004) The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. J Neurol 251:261–268
LeVine SM (1992) The role of reactive oxygen species in the pathogenesis of multiple sclerosis. Med Hypotheses 39:271–274
LeVine SM, Wetzel DL (1998) Chemical analysis of multiple sclerosis lesions by FT-IR microspectroscopy. Free Radic Biol Med 25:33–41
Naidoo R, Knapp ML (1992) Studies of lipid peroxidation products in cerebrospinal fluid and serum in multiple sclerosis and other conditions. Clin Chem 38:2449–2454
Greco A, Minghetti L, Levi G (2000) Isoprostanes, novel markers of oxidative injury, help understanding the pathogenesis of neurodegenerative diseases. Neurochem Res 25:1357–1364
McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, McFarland HF, Paty DW, Polman CH, Reingold SC, Sandberg-Wollheim M, Sibley W, Thompson A, van den Noort S, Weinshenker BY, Wolinsky JS (2001) Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 50:121–127
Cherif H, Tarry JL, Ozanne SE, Hales CN (2003) Ageing and telomeres: a study into organ- and gender-specific telomere shortening. Nucleic Acids Res 31:1576–1583
de Lange T, Shiue L, Myers RM, Cox DR, Naylor SL, Killery AM, Varmus HE (1990) Structure and variability of human chromosome ends. Mol Cell Biol 10:518–527
Esterbauer H, Striegl G, Puhl H, Rotheneder M (1989) Continuous monitoring of in vitro oxidation of human low density lipoprotein. Free Radic Res Commun 6:67–75
Gilgun-Sherki Y, Melamed E, Offen D (2001) Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 40:959–975
Rokach J, Khanapure SP, Hwang S-W, Adiyaman M, Lawson JA, FitzGerald GA (1997) The isoprostanes: a perspective. Prostaglandins 54:823–851
Greco A, Minghetti L, Sette G, Fieschi C, Levi G (1999) Cerebrospinal fluid isoprostane shows oxidative stress in patients with multiple sclerosis. Neurology 53:1876–1879
Besler HT, Comoglu S (2003) Lipoprotein oxidation, plasma total antioxidant capacity and homocysteine level in patients with multiple sclerosis. Nutr Neurosci 6:189–196
Ferretti G, Bacchetti T, Principi F, Di Ludovico F, Viti B, Angeleri VA, Danni M, Provinciali L (2005) Increased levels of lipid hydroperoxides in plasma of patients with multiple sclerosis: a relationship with paraoxonase activity. Mult Scler 11:677–682
Bassett CN, Montine TJ (2003) Lipoproteins and lipid peroxidation in Alzheimer’s disease. J Nutr Health Aging 7:24–29
Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, Greider CW, Harley CB (1992) Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA 89:10114–10118
Dandona P, Dhindsa S, Aljada A, Chaudhuri A (2004) Classical anti-oxidants (Scavengers) versus biological anti-oxidants (suppressors of ROS generation): a novel way to explain the anti-oxidant paradox. Metab Syndr Relat Disord 2:155–159
Aviv A (2004) Telomeres and human aging: facts and fibs. Sci Aging Knowledge Environ 51:pe43
Vladimirova O, Lu FM, Shawver L, Kalman B (1999) The activation of protein kinase C induces higher production of reactive oxygen species by mononuclear cells in patients with multiple sclerosis than in controls. Inflamm Res 48:412–416
von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27:339–344
Hug A, Korporal M, Schroder I, Haas J, Glatz K, Storch-Hagenlocher B, Wildemann B (2003) Thymic export function and T cell homeostasis in patients with relapsing remitting multiple sclerosis. J Immunol 171:432–437
Newcombe J, Li H, Cuzner ML (1994) Low density lipoprotein uptake by macrophages in multiple sclerosis plaques: implications for pathogenesis. Neuropathol Appl Neurobiol 20:152–162
Acknowledgments
This work was supported by grants from the National Natural. Science Fund (NSFC) (81170329/H2501), and 2012 Health and Labour Sciences Research Grants Comprehensive Research on Life-Style Related Diseases including Cardiovascular Diseases and Diabetes Mellitus, and the Ministry of Education, Science, and Culture of Japan (#23590885).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jing-Zhi Guan, Wei-Ping Guan and Toyoki Maeda have equally contributed to this work.
Rights and permissions
About this article
Cite this article
Guan, JZ., Guan, WP., Maeda, T. et al. Patients with multiple sclerosis show increased oxidative stress markers and somatic telomere length shortening. Mol Cell Biochem 400, 183–187 (2015). https://doi.org/10.1007/s11010-014-2274-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-2274-1