Abstract
Purpose
The Hospital Frailty Risk Score (HFRS) is derived from routinely collected data and validated as a geriatric risk stratification tool. This study aimed to evaluate the utility of the HFRS as a predictor for postoperative adverse events in spine surgery.
Methods
In this retrospective analysis of 2042 patients undergoing spine surgery at a university spine center between 2011 and 2019, HFRS was calculated for each patient. Multivariable logistic regression models were used to assess the relationship between the HFRS and postoperative adverse events. Adverse events were compared between patients with high or low frailty risk.
Results
Patients with intermediate or high frailty risk showed a higher rate of reoperation (19.7% vs. 12.2%, p < 0.01), surgical site infection (3.4% vs. 0.4%, p < 0.001), internal complications (4.1% vs. 1.1%, p < 0.01), Clavien–Dindo IV complications (8.8% vs. 3.4%, p < 0.001) and transfusion (10.9% vs. 1.5%, p < 0.001). Multivariable logistic regression analyses revealed a high HFRS as independent risk factor for reoperation [odds ratio (OR) = 1.1; 95% confidence interval (CI) 1.0–1.2], transfusion (OR = 1.3; 95% CI 1.2–1.4), internal complications (OR = 1.2; 95% CI 1.1–1.3), surgical site infections (OR = 1.3; 95% CI 1.2–1.5) and other complications (OR = 1.3; 95% CI 1.2–1.4).
Conclusion
The HFRS can predict adverse events and is an easy instrument, fed from routine hospital data. By identifying risk patients at an early stage, the individual patient risk could be minimized, which leads to less complications and lower costs.
Level of evidence
Level III – retrospective cohort study
Trial registration
The study was approved by the local ethics committee (20-1821-104) of the University of Regensburg in February 2020.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to the ageing of the world population, the number of degenerative spinal diseases requiring surgical intervention grows steadily. Geriatric patients demand rapid therapeutic success and preservation of their quality of life [1, 2]. Along with the surgical advances, spine surgery in older patients has increased over time.[3, 4]. As geriatric patients have higher perioperative complication rates, it is important to have an estimation of the patient’s individual risk, due to the substantial biological differences in people of the same age [5,6,7]. Internal concomitant diseases such as cerebrovascular diseases, heart–lung diseases, and kidney problems, which predominantly occur in geriatric patients, can also have a negative influence on the postoperative results [8]. Besides the quality aspect, poor outcomes and adverse events with prolonged hospitalization are also a socio-economic burden for the public health system all over the world [9]. Thus, practitioners are forced to further outcome optimization and risk stratification due to socio-economic aspects. Reducing complications means perioperative recognition and optimization of modifiable risk factors [10, 11].
Therefore, frailty is becoming increasingly important in the surgical field to identify people at high risk of poor outcomes [12,13,14]. Physical frailty has been defined in 2013 by members of a consensus group of delegates from 6 major international, European, and US societies as: “A medical syndrome with multiple causes and contributors that is characterized by diminished strength, endurance, and reduced physiologic function that increases an individual’s vulnerability for developing increased dependency and/or death” [15]. Many tools have been developed to stratify risk or frailty. The Hospital Frailty Risk Score (HFRS), published and validated in 2018 by Gilbert et al., acts as well or often even better than existing tools for risk stratification or frailty assessment. The great advantage of the HFRS to other systems is that it can be derived at any time from the existing data of the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes, and thus everywhere where the ICD-10 coding system is used the HFRS can be applied without additional data acquisition. In addition, the HFRS can be implemented in most hospital information systems at low cost, avoiding the interoperator variability and implementation burden associated with manual scores [16].
A recent review found 8 studies showing frailty associated with higher risk of morbidity and mortality in patients receiving spine surgery [17]. Only one of them used the HFRS, but without analyzing adverse events in detail [18]. Our study group has already shown the advantage using HFRS to predict adverse outcomes in hip and knee arthroplasty, but there is not enough information regarding the effect in predicting adverse outcomes in spine surgery [11, 19].
The aim of our study was to evaluate the utility of the HFRS as a predictor for postoperative adverse events after spine surgery.
Methods
Study design and study population
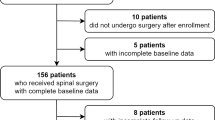
In this retrospective study, a dataset derived from the department’s spine surgery registry and the hospital information system was used. The study was approved by the local ethics committee (20-1821-104) of the University of Regensburg. All methods were carried out in accordance with the ethical standards of the Declaration of Helsinki 1975. As it is retrospective study the need of an informed consent was waived by the ethical committee of the University of Regensburg. From the database of our high-volume orthopedic university department with an extra spinal unit, all patients who underwent lumbar spine surgery (fusion/decompression) for degenerative reasons between 2011 and 2019 were included. Exclusion criteria were former spine surgery, infection, trauma, and tumors or patients with incomplete data files.
The investigation used a power calculation of the primary end point of reoperation within 90 days after spine surgery. As with revision procedures, all surgeries that required anesthesia and which were done in the OR, such as deep wound infects, screw loosening or displacement and cerebrospinal fluid (CSF) leak were included. The hypothesis was tested with a significance level of 5%. The expected difference in complications was set conservatively to 7% referring to a previous study [11]. To achieve a power of 80% using 2-sample chi-square test (nQuery Advisor 7.0, Statistical Solutions Ltd, Cork, Ireland), a sample size of 620 in the low-risk group and of 124 in the high-risk group assuming a 5:1 ratio was calculated. Complications and transfusion were set as secondary end points. As in the former studies, complications were categorized into surgical (surgical site infection), internal (myocardial infarction, acute heart failure, cardiac arrhythmias, pneumonia, renal failure, electrolyte imbalance), and other complications (collapse, thrombosis, pulmonary embolism, urinary tract infection, delirium, stroke) [11].
The Clavien–Dindo classification was also used to categorize complications, which divides complications into 5 groups upon the therapy need for correction [20]. A grade I complication is one in which any deviation from the normal postoperative course without the need for drug treatment or surgical, endoscopic, and radiological intervention. Grade II complications require specific pharmacological treatment. Grade III complications result in the necessity of surgical, endoscopic, or radiological intervention. Grade IV complications are defined as life-threatening events that require treatment in intensive care units. When a patient dies its graded V [20].
Data collection
Principal and secondary diagnostic codes including the corresponding ICD-10 codes were entered by professional clinical coders in the hospital information system (ORBIS; Agfa Healthcare) and then extracted and double-checked by physicians using the information medical records information. Other clinical data recollected from the register were age, gender, length of stay, transfusion, transfer to the intensive care unit, reoperation, readmission, and complications.
Calculation of the HFRS
The HFRS was calculated retrospectively with help from the ICD-10 codes that were entered at admission and from previous stays. The HFRS derives from 109 ICD-10 codes that were identified as characteristic of a cluster of frail individuals. Different points were awarded to each code and summed up to a maximum possible score of 173.2 points, depending on how strong each ICD-10 code predicted membership in the cluster of frail patients. Gilbert et al. classified frailty into three risk categories, low HFRS under 5-point, intermediate HFRS between 5 and 15 points, and high HFRS above 15 points. Weighting factors and ICD-10 codes for the HFRS according to the literature are provided in the appendix (Appendix 1) [16].
Statistics
Continuous data was stated as mean (standard deviation) for statistical analysis. Two-sided t-test was applied for group comparisons. For categorical data were absolute and relative frequencies, which were then compared by chi-square tests between groups. The hypothesis of the study was tested with a significance level of 5%. To assess whether HFRS is a significant predictor of reoperation, readmission, and complications while controlling for demographic variables known to be associated with adverse surgical outcomes such as age, sex, and the American Society of Anesthesiologists classification (ASA) a multivariable logistic regression analysis was performed [21]. For the statistical analysis IBM SPSS Statistics 25 (SPSS Inc, Chicago, IL) was used.
Results
During the studied period from January 2011 to December 2019, 2042 patients were identified who underwent spine surgery. In Table 1, the demographic characteristics of the study group are presented. The mean HFRS in the study group was 1.5 ± 2.2. As low risk (HFRS < 5), 93% (1895/2042) of the patients were categorized 7.1% (145/2042) as intermediate risk (HFRS 5–15), and 0.1% (2/2042) as high risk (HFRS > 15). In Fig. 1, the HFRS distribution in the study group is shown. The intermediate and high-risk groups were pooled for further analysis, due to the limited number of high-risk patients in the study group. With increasing the HFRS, the number of all measured adverse events increased (Figs. 2 and 3). The relation between fusion and non-fusion was 64–36%. All fusion were done from posterior (TLIF or PLIF). There was not significant difference between these 2 groups regarding the examined parameters.
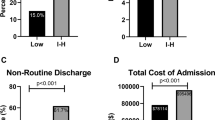
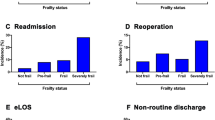
The overall revision surgery rate in all groups within 90 days after spine surgery was 12.2% (260/2042). In the intermediate/high frailty risk group the risk of revision surgery within 90 days was 19.7%, that means 7.5% higher than in the low risk of frailty group (12.2%).
Regarding the Clavien–Dindo grade IV complications, there was a complication rate of 3.8% (78/2042) in total. The complication rate in the intermediate/high frailty risk group was 8.8%, that means 5.4% higher than in the low risk of frailty group (3.4%).
Furthermore, the overall transfusion rate was 2.2% (45/2042). In the intermediate/high frailty risk group, the transfusion rate was 10.9%, that means 9.4% higher than in the low risk of frailty group (1.5%).
Internal complications in total were 1.3% (27/2042). The internal complications were 4.1% and thus 3% higher in the intermediate/high frailty risk group than in the low risk of frailty group (1.1%).
Taking the occurrence of other complications (thromboembolisms, apoplexy, delirium, syncope, and collapse) into account, the total rate was 2.6% (53/2042). The complication rate in the intermediate/high frailty risk group was 12.9%, 11.1% higher than in the low risk of frailty group (1.8%).
In total, the surgical site infection rate was 0.6% (13/2042). The rate was 3.0% higher in the intermediate/high frailty risk group (3.4%) than in the low risk of frailty group (0.4%) (Table 2).
In addition, the surgical time (minutes) (148 ± 132/118 ± 107; p = 0.001) and length of stay (LOS, days) (13 ± 10/8 ± 6; p < 0.001) were significantly longer in the intermediate/high frailty risk group than in the low risk of frailty group.
Multivariable logistic regression analysis
Revision rate within the first 90 days
The revision rate within the first 90 days after spine surgery showed an independent association with HFRS [odds ratio (OR) = 1.1; 95% confidence interval (CI) 1.0–1.2; p = 0.001] as well as surgical time (OR = 1.0; 95% CI 1.0–1.0; p < 0.001) and ASA classification (OR = 1.5; 95% CI 1.2–1.2; p < 0.001), but not with age or gender.
Clavien–Dindo IV complications
The Clavien–Dindo IV complications were not associated with the HFRS (OR = 1.1; 95% CI 1.0–1.2; p = 0.2), but long surgical time (OR = 1.0; 95% CI 1.0–1.0; p < 0.001) and high ASA classifications were independent risk factors for complications requiring intensive care unit (ICU) management (OR = 2.4; 95% CI 1.6–3.7; p < 0.001).
Transfusion
The HFRS was an independent risk factor for transfusion (OR = 1.29; 95% CI 1.2–1.4; p < 0.001) as well as age (OR = 1.0; 95% CI 1.0–1.0; p < 0.001), ASA (OR = 2.3; 95% CI 1.3–4.0; p = 0.002) and surgical time (OR = 1.0; 95% CI 1.0–1.0; p < 0.001).
Internal complications
The HFRS (OR = 1.2; 95% CI 1.1–1.3; p = 0.003), and age (OR = 1.1; 95% CI 1.0–1.1, p < 0.001) were independent risk factors for internal complications. However, there was no correlation with the ASA classification (OR = 0.6; 95% CI 0.6–2.6; p = 0.6).
Other complications
Similarly, HFRS (OR = 1.3; 95% CI 1.2–1.4; p < 0.001) and age (OR = 1.0; 95% CI 1.0–1.1; p = 0.004), but not ASA classification (OR = 0.7; 95% CI 0.4–1.1; p = 0.2) were independent risk factor thromboembolisms, apoplexy, delirium, syncope and collapse.
Surgical site infection
There was an association between surgical site infection and HFRS (OR = 1.3; 95% CI 1.2–1.5; p < 0.001), and age (OR = 1.0; 95% CI 0.9–1.0; p = 0.004), but not with ASA classification (OR = 1.5; 95% CI 0.6–3.6; p < 0.001) and surgical time (OR = 1.0; 95% CI 1.0–1.0; p = 0.6).
Gender
Gender showed no association with all the parameters measured.
Discussion
This study aims to validate the HFRS regarding adverse events after primary spine surgery. To reduce adverse events and associated treatment costs, in the last years, several risk assessment tools focusing on comorbidities have been introduced and evaluated [10, 11]. Ideally, these tools allow doctors and patients to carry out a risk assessment in advance of an operation in order to understand risks associated with the operation and facilitate obtaining informed consent.
Recently, the concept of frailty has become increasingly important in terms of risk stratification and outcome prediction [21, 22]. Although there are some other studies, which investigated the concept of frailty as a predictor of surgical outcome in patients undergoing spine surgery, to the best of our knowledge, the current study is the first to investigate the relation of HFRS and Clavien–Dindo classification, internal (cardio-pulmonal, thrombosis, transfusion, etc.), other complications (thromboembolisms, apoplexy, delirium, syncope and collapse) or surgical site infections in spine surgery [23].
Frailty is defined as a multidimensional geriatric syndrome that can be triggered by only minor disruptive factors and leads to sudden changes in the state of health [15]. The loss of physical and mental reserves is typical [24]. In the literature, poor outcomes after surgery and higher rates of adverse postoperative events are associated with increased frailty [11]. In 2018, Gilbert et al. published the HFRS with the idea of cumulative deficits in frailty. He intended to use routinely collected administrative data to develop a screening tool for frailty that can be used anywhere at low cost. In its validation study, the HFRS showed good agreement with popular frailty scales (i.e., Fried Phenotype, Rockwood's Frailty Index). The goal of identifying patients at higher risk for mortality, longer length of stay, and readmission was achieved.
In our study, the patients were treated as described by Gilbert et al. and divided into 2 cohorts: a cohort with a low risk of frailty and a cohort with intermediate or high risk of frailty were formed. In the original study, the mean age was 84 years and mean HFRS was 9 [16]. In our orthopedic setting and due to mostly elective surgery, the mean HFRS was 1.5 and mean age was 60. 7.2% of the patients were in the intermediate or high risk of frailty group. Despite these different characteristics and a younger patient cohort, a group with higher risk of adverse outcomes could be identified. In comparison with another study of our group including patients with hip and knee arthroplasty (mean HFRS 0.9, 4.7% intermediate or high risk), the spine cohort had a higher mean HFRS [11]. The reason might be that despite the elective character of orthopedic operations, some spinal operations are more urgent than hip or knee arthroplasty and thus patients in worse conditions.
In 2020, Hannah et al. published a correlation of HFRS with age, gender, ASA classification, Elixhauser Index, revision surgery, fusion rate, median number of segments fused, estimated blood loss and length of surgery.
In our study, the rate of revision surgery within 90 days was 1.6× higher for the intermediate/high risk of frailty patients. Hannah et al. saw also a good prediction for surgical complications, but only a weak effect on 30-day readmission. The explanation might be that in patients with higher risk of frailty, surgical complications occur within the initial hospital stay and revision surgery occurs immediately without discharge in between. This explanation is supported by our results that the LOS in the intermediate/high frailty risk group is significantly longer. The multivariable logistic regression showed an independent association of HFRS, surgical time, and ASA classification with the revision surgery rate.
ASA classification and long surgical time also seem to be better predictive parameters for complications requiring an ICU stay (Clavien–Dindo IV), than HFRS. Although the high and intermediate risk of frailty group had a 2.5× higher risk for Clavien–Dindo IV complications, there was no association in the multivariable logistic regression analysis. Hannah et al., in comparison, described an improved accuracy of predicting ICU stays in the logistic regression analysis for HFRS [18]. The reason might be the rate of 11.7% compared to 4.7% in our cohort for higher and intermediate risk of frailty patients. Different studies for knee and hip arthroplasty confirmed our results; that there is, on the one hand a higher risk for ICU stay, but no independent association of the HFRS [11]. Although, in a different frailty scoring system, the modified Frailty index (mFI) based on the Canadian Study of Health and Ageing Frailty Index, the multivariable analysis showed that the preoperative mFI and ASA classification of ≥ III had a significantly increased risk of leading to Clavien IV complications and death, the HFRS might be less suitable for prediction of ICU admission as potentially life-threatening comorbidity [23]. Bruno et al. also confirmed in 2019 that there is only a limited predictive value of the HFRS in ICU patients [25].
In the high and intermediate risk of frailty group, the transfusion rate was 7.2× higher, an independent association could only be seen for the ASA classification, but not for HFRS. Identifying these patients at an early stage seems to be important. Recent studies have shown that a patient blood management strategy 4 weeks before surgery is effective to decrease the rate of transfusion in risk patients [26].
The most important finding of this study is that the HFRS showed an independent association with non-life-threatening complications, but with the need to treat, such as cardio-pulmonal or renal complications, thromboembolisms, apoplexy, delirium, syncope and collapse. In comparison, there was no association for these parameters for ASA classification. Despite the fact that ICD-10 codes for cardiovascular and pulmonary diseases are not included in the HFRS, the rate for cardio-pulmonal complications was 3.7× higher and for thromboembolisms, apoplexy, delirium, syncope and collapse even 7× higher in the high and intermediate risk of frailty group [8]. To improve the predictive value of HFRS, it should be modified in future studies using specific ICD-10 codes [27].
Regarding the surgical site infection rate, HFRS is also independently associated. In the intermediate and high risk of frailty group, the infection rate is 8.5× higher than in the low-risk rate. As in spinal surgery the incidence of infection is higher than in general orthopedic operations, the HFRS can be a simple predictor. Recently, a scoring system was published with the single aim to identify the risk patients to reduce complex and expensive infection treatments and thereby reducing costs in the healthcare system [28].
Our findings show that using the ASA classification, severe complications with the need for ICU treatment could be predicted, but by using the HFRS and not the ASA classification, many complications without the need of an ICU could be avoided, by identifying those patients and giving them special attention and treatment before surgery. Particularly, “milder” complications can often be easily and simply avoided by patient education and optimizing modifiable risk factors preoperatively, but if not, they are the reason for prolonged hospital stay and negative socio-economic effects [29].
There are several limitations like in most database studies. The HFRS derives from ICD-10 codes, therefore the accuracy of coding is a potential source of bias. Additionally, the only available data came from the hospital information system and spine registry. Therefore, parameters such as body-mass index or psychosocial factors, known as outcome influencing, had to be excluded because of the lack of data. In addition, the parameter “length of stay” has been seen in the context of the German health care system with usually a longer length of stay internationally compared. The preoperative counselling and preoperative assessment process has an implication on the distribution in the study group. This may have a bearing on the recommendations/conclusions from/of the study due to fairly small numbers in the intermediate/high risk group and the retrospective nature of the study.
Another limitation is that due to the routine data, the number of levels of fusion or decompression could not exactly be determined and therefore, the severity of the surgery might be confounder. On the other hand, that means that the results can be transferred to a wide range of spine operations and not only to a group defined by strict in- and exclusion criteria.
Despite these limitations, this study is the first to demonstrate the predictive value of HFRS in patients with spine surgery.
The strength of our study is the single center design that guarantees standardized operative workflows and postoperative treatment protocols for all spine operations. Another possible bias by using different fusion systems could be avoided by the supply of only one manufacturer. That means a reduction of possible confounding factors.
Conclusion
In our study, we were able to show that the HFRS can predict adverse events such as reoperation, complications and surgical site infections after spine surgery. As an inexpensive instrument that is fed from routine data in the hospital database, it can identify risk patients at an early stage. Thus, the individual patient risk could be minimized in advance through optimization of modifiable risk factors. This reduces the socio-economic burden and increases patient safety.
Data availability
On request.
References
Nimptsch U, Bolczek C, Spoden M et al (2018) Volume growth of inpatient treatments for spinal disease-analysis of German nationwide hospital discharge data from 2005 to 2014. Z Orthop Unfall 156:175–183. https://doi.org/10.1055/s-0043-119898
Benditz A, Grifka J (2019) Lumbar spinal stenosis: from the diagnosis to the correct treatment. Orthopade. https://doi.org/10.1007/s00132-018-03685-3
Wieser LM, Sauermann S, Weber F (2016) How many cases of spine surgery are performed in Germany? method of counting the number of cases of spine surgery in Germany. J Neurol Surgery Part A Cent Eur Neurosurg. https://doi.org/10.1055/s-0035-1570000
Martin BI, Mirza SK, Spina N et al (2019) Trends in lumbar fusion procedure rates and associated hospital costs for degenerative spinal diseases in the United States, 2004 to 2015. Spine. https://doi.org/10.1097/BRS.0000000000002822
Miller EK, Neuman BJ, Jain A et al (2017) An assessment of frailty as a tool for risk stratification in adult spinal deformity surgery. Neurosurg Focus. https://doi.org/10.3171/2017.10.FOCUS17472
Muhl A, Teßmann R (2004) Periphere regionalanästhesien der oberen extremität. Trauma und Berufskrankheit 6:117–125. https://doi.org/10.1007/s10039-004-0876-7
Phan K, Kim JS, Somani S et al (2018) Impact of age on 30-day complications after adult deformity surgery. Spine. https://doi.org/10.1097/BRS.0000000000001832
Lee MJ, Hacquebord J, Varshney A et al (2011) Risk factors for medical complication after lumbar spine surgery: a multivariate analysis of 767 patients. Spine. https://doi.org/10.1097/BRS.0b013e318219d28d
Barnett ML, Wilcock A, McWilliams JM et al (2019) Two-year evaluation of mandatory bundled payments for joint replacement. N Engl J Med. https://doi.org/10.1056/nejmsa1809010
Harari D, Hopper A, Dhesi J et al (2007) Proactive care of older people undergoing surgery (‘POPS’): designing, embedding, evaluating and funding a comprehensive geriatric assessment service for older elective surgical patients. Age Ageing. https://doi.org/10.1093/ageing/afl163
Meyer M, Parik L, Leiß F et al (2020) Hospital frailty risk score predicts adverse events in primary total hip and knee arthroplasty. J Arthroplast 35:3498-3504.e3. https://doi.org/10.1016/j.arth.2020.06.087
Acosta FL, McClendon J, O’Shaughnessy BA et al (2011) Morbidity and mortality after spinal deformity surgery in patients 75 years and older: complications and predictive factors: clinical article. J Neurosurg Spine. https://doi.org/10.3171/2011.7.SPINE10640
Hubbard RE, Peel NM, Samanta M et al (2017) Frailty status at admission to hospital predicts multiple adverse outcomes. Age Ageing. https://doi.org/10.1093/ageing/afx081
Buurman BM, Van Den Berg W, Korevaar JC et al (2011) Risk for poor outcomes in older patients discharged from an emergency department: feasibility of four screening instruments. Eur J Emerg Med 18:215–220
Morley JE, Vellas B, Abellan van Kan G et al (2013) Frailty consensus: a call to action. J Am Med Dir Assoc. https://doi.org/10.1016/j.jamda.2013.03.022
Gilbert T, Neuburger J, Kraindler J et al (2018) Development and validation of a hospital frailty risk score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. https://doi.org/10.1016/S0140-6736(18)30668-8
Chan V, Wilson JRF, Ravinsky R et al (2021) Frailty adversely affects outcomes of patients undergoing spine surgery: a systematic review. Spine J. https://doi.org/10.1016/j.spinee.2021.01.028
Hannah TC, Neifert SN, Caridi JM et al (2020) Utility of the hospital frailty risk score for predicting adverse outcomes in degenerative spine surgery cohorts. Neurosurgery. https://doi.org/10.1093/neuros/nyaa248
Meyer M, Parik L, Greimel F et al (2020) Hospital frailty risk score outperforms current risk stratification models in primary total hip and knee arthroplasty. J Arthroplast. https://doi.org/10.1016/j.arth.2020.12.002
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205
Shin JI, Keswani A, Lovy AJ, Moucha CS (2016) simplified frailty index as a predictor of adverse outcomes in total hip and knee arthroplasty. J Arthroplast. https://doi.org/10.1016/j.arth.2016.04.020
Eamer G, Al-Amoodi MJH, Holroyd-Leduc J et al (2018) Review of risk assessment tools to predict morbidity and mortality in elderly surgical patients. Am J Surg 216:585–594
Ali R, Schwalb JM, Nerenz DR et al (2016) Use of the modified frailty index to predict 30-day morbidity and mortality from spine surgery. J Neurosurg Spine. https://doi.org/10.3171/2015.10.SPINE14582
Clegg A, Young J, Iliffe S et al (2013) Frailty in elderly people. The Lancet 381:752–762
Bruno RR, Wernly B, Flaatten H et al (2019) The hospital frailty risk score is of limited value in intensive care unit patients. Crit Care 23:1–2
Kang T, Park SY, Nam JJ et al (2019) Patient blood management during lumbar spinal fusion surgery. World Neurosurg. https://doi.org/10.1016/j.wneu.2019.06.153
Lakomkin N, Zuckerman SL, Stannard B et al (2019) Preoperative risk stratification in spine tumor surgery: a comparison of the modified Charlson index, frailty index, and ASA score. Spine. https://doi.org/10.1097/BRS.0000000000002970
Namba T, Ueno M, Inoue G et al (2020) Prediction tool for high risk of surgical site infection in spinal surgery. Infect Control Hosp Epidemiol. https://doi.org/10.1017/ice.2020.107
Kwok CS, Zieroth S, Van Spall HGC et al (2020) The hospital frailty risk score and its association with in-hospital mortality, cost, length of stay and discharge location in patients with heart failure short running title: frailty and outcomes in heart failure. Int J Cardiol. https://doi.org/10.1016/j.ijcard.2019.09.064
Acknowledgements
We are grateful to L. Quintana and R. Pulido for their technical assistance.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
LCP, MM, JR, TK, JG and MW made substantial contributions to the conception and design of the study. LCP, MM, JR and MW participated in the acquisition of data, analysis and statistics. All authors made contributions to the interpretation of data and have been involved in drafting the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Consent to participate
As it is retrospective study the need of an informed consent was waived by the ethical committee of the University of Regensburg.
Consent for publication
All authors give their consent to publish this article in the ESJ.
Ethics approval
The study was approved by the local ethics committee (20-1821-104) of the University of Regensburg. All methods were carried out in accordance with the ethical standards of the Declaration of Helsinki 1975.
Additional information
Communicated by GERMANY.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pulido, L.C., Meyer, M., Reinhard, J. et al. Hospital frailty risk score predicts adverse events in spine surgery. Eur Spine J 31, 1621–1629 (2022). https://doi.org/10.1007/s00586-022-07211-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-022-07211-0