Abstract
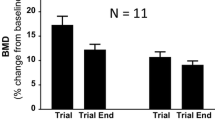
A case series of six women with postmenopausal osteoporosis who had received continuous denosumab for 7 years and were then given a single infusion of zoledronate (5 mg) is reported. During denosumab treatment, bone mineral density (BMD) in the spine increased 18.5% (P = 0.006), and total hip BMD by 6.9% (P = 0.03). Post-zoledronate BMDs were measured 18–23 months after treatment, and there were significant declines at each site (P spine = 0.043, P hip = 0.005). Spine BMD remained significantly above the pre-denosumab baseline (+9.3%, P = 0.003), but hip BMD was not significantly different from baseline (−2.9%). At the time of post-zoledronate BMD measurements, serum PINP levels were between 39 and 60 μg/L (mean 52 μg/L), suggesting that the zoledronate treatment had not adequately inhibited bone turnover. It is concluded that this regimen of zoledronate administration is not adequate to preserve the BMD gains that result from long-term denosumab treatment.

Similar content being viewed by others
References
Bone HG, Bolognese MA, Yuen CK et al (2011) Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab 96:972–980
Miller PD, Bolognese MA, Lewiecki EM et al (2008) Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone 43:222–229
Popp AW, Buffat H, Senn C et al (2016) Rebound-associated bone loss after non-renewal of long-term denosumab treatment offsets 10-year gains at the total hip within 12 months. J Bone Miner Res 31(suppl):S408
Popp AW, Zysset PK, Lippuner K (2016) Rebound-associated vertebral fractures after discontinuation of denosumab—from clinic and biomechanics. Osteoporos Int 27:1917–1921
Aubry-Rozier B, Gonzalez-Rodriguez E, Stoll D et al (2016) Severe spontaneous vertebral fractures after denosumab discontinuation: three case reports. Osteoporos Int 27:1923–1925
Anastasilakis AD, Polyzos SA, Makras P et al (2017) Clinical features of 24 patients with rebound-associated vertebral fractures following denosumab discontinuation: systematic review and additional cases. J Bone Miner Res. doi:10.1002/jbmr.3110
Anastasilakis AD, Makras P (2016) Multiple clinical vertebral fractures following denosumab discontinuation. Osteoporos Int 27:1929–1930
Lamy O, Gonzalez-Rodriguez E, Stoll D et al (2017) Severe rebound-associated vertebral fractures after denosumab discontinuation: 9 clinical cases report. J Clin Endocrinol Metab 102:354–358
Freemantle N, Satram-Hoang S, Tang ET et al (2012) Final results of the DAPS (denosumab adherence preference satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int 23:317–326
Cummings SR, San Martin J, McClung MR et al (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756–765
Papapoulos S, Lippuner K, Roux C et al (2015) The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM Extension study. Osteoporos Int 26:2773–2783
Grey A, Bolland MJ, Horne A et al (2012) Five years of anti-resorptive activity after a single dose of zoledronate—results from a randomized double-blind placebo-controlled trial. Bone 50:1389–1393
McClung M, Miller P, Recknor C et al (2009) Zoledronic acid for the prevention of bone loss in postmenopausal women with low bone mass a randomized controlled trial. Obstet Gynecol 114:999–1007
Grey A, Bolland M, Wattie D et al (2010) Prolonged antiresorptive activity of zoledronate: a randomized, controlled trial. J Bone Miner Res 25:2251–2255
Acknowledgement
This study was supported by the Health Research Council of New Zealand.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ian R. Reid has received research grants and honoraria from Amgen, Novartis, Merck, and Lilly. Anne M. Horne, Borislav Mihov, and Gregory D.Gambie have nothing to declare.
Human and Animal Rights and Informed Consent
The FREEDOM study and its extensions were approved by our regional ethics committee.
Rights and permissions
About this article
Cite this article
Reid, I.R., Horne, A.M., Mihov, B. et al. Bone Loss After Denosumab: Only Partial Protection with Zoledronate. Calcif Tissue Int 101, 371–374 (2017). https://doi.org/10.1007/s00223-017-0288-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-017-0288-x