Abstract
Summary
Adherence and persistence to oral bisphosphonates in women with postmenopausal osteoporosis is suboptimal. In this study, patients were treated with either oral or intravenous bisphosphonates. The increased adherence and persistence observed in patients receiving intravenous medication compared with those receiving oral medication may improve health outcomes.
Introduction
Poor adherence and persistence to oral medication are often observed in women with postmenopausal osteoporosis (PMO). The purpose of the non-interventional BonViva Intravenous Versus Alendronate (VIVA) study was to determine whether, in a real-world setting, (1) increased adherence and persistence to medication would be observed in women with PMO receiving intravenous (i.v.) ibandronate versus oral alendronate, (2) a correlation exists between adherence and persistence to medication and drug efficacy, and (3) any unexpected adverse events/serious adverse events (AEs/SAEs) may occur.
Methods
The study was conducted in 632 centers in Germany. A total of 6,064 females with PMO were enrolled and recruited into one of two treatment arms: quarterly i.v. administration of 3 mg ibandronate or weekly oral medication of 70 mg alendronate, for 12 months. At the end of the study, adherence and persistence to medication, new osteoporotic fractures, mobility, use of analgesics, and AEs/SAEs were determined.
Results
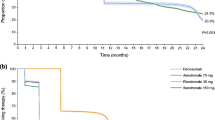
Greater adherence and persistence to medication were observed in the ibandronate treatment arm compared with the alendronate treatment arm. Although there was no significant difference in the number of patients with new vertebral, hip, or forearm fractures between treatment arms, a significantly greater increase in mobility and decrease in the use of analgesics were reported in the ibandronate treatment arm. No unexpected AEs/SAEs occurred in either arm.
Conclusions
Adherence and persistence to medication were greater in women with PMO receiving i.v. ibandronate compared with those receiving oral alendronate. This may have led to an increase in mobility and a decrease in pain in these patients.



Similar content being viewed by others
References
National Osteoporosis Foundation (2003) Physician’s guide to prevention and treatment of osteoporosis. Excerpta Medica, Belle Mead
Sambrook P, Cooper C (2006) Osteoporosis. Lancet 367:2010–2018
Hadji P, Klein S, Gothe H, Häussler B, Kless T, Schmidt T, Steinle T, Verheyen F, Linder R (2013) The epidemiology of osteoporosis—Bone Evaluation Study (BEST): an analysis of routine health insurance data. Dtsch Arztebl Int 110:52–57
Ström O, Borgström F, Kanis JA, Compston J, Cooper C, McCloskey EV, Jönsson B (2012) Osteoporosis: burden, health care provision and opportunities in the EU: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 6:59–155
Hooven FH, Adachi JD, Adami S, Boonen S, Compston J, Cooper C, Delmas P, Diez-Perez A, Gehlbach S, Greenspan SL, LaCroix A, Lindsay R, Netelenbos JC, Pfeilschifter J, Roux C, Saag KG, Sambrook P, Silverman S, Siris E, Watts NB, Anderson FA Jr (2009) The Global Longitudinal Study of Osteoporosis in Women (GLOW): rationale and study design. Osteoporos Int 20:1107–1116
Prieto-Alhambra D, Nogues X, Javaid MK, Wyman A, Arden NK, Azagra R, Cooper C, Adachi JD, Boonen S, Chapurlat RD, Compston JE, Gehlbach SH, Greenspan SL, Hooven FH, Netelenbos JC, Pfeilschifter J, Rossini M, Sambrook PN, Silverman S, Siris ES, Watts NB, Díez-Pérez A (2013) An increased rate of falling leads to a rise in fracture risk in postmenopausal women with self-reported osteoarthritis: a prospective multinational cohort study (GLOW). Ann Rheum Dis 72(6):911–7
Rizzoli R (2011) Bisphosphonates for post-menopausal osteoporosis: are they all the same? QJM 104:281–300
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348:1535–1541
Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH 3rd, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. JAMA 282:1344–1352
Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, Dobbels F, Fargher E, Morrison V, Lewek P, Matyjaszczyk M, Mshelia C, Clyne W, Aronson JK, Urquhart J, ABC Project Team (2012) A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol 73:691–705
Caro JJ, Ishak KJ, Huybrechts KF, Raggio G, Naujoks C (2004) The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int 15:1003–1008
Höer A, Seidlitz C, Gothe H, Schiffhorst G, Olson M, Hadji P, Häussler B (2009) Influence on persistence and adherence with oral bisphosphonates on fracture rates in osteoporosis. Patient Prefer Adherence 3:25–30
Penning-van Beest FJ, Erkens JA, Olson M, Herings RM (2008) Loss of treatment benefit due to low compliance with bisphosphonate therapy. Osteoporos Int 19:511–517
Siris ES, Selby PL, Saag KG, Borgström F, Herings RM, Silverman SL (2009) Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am J Med 122(2 Suppl):S3–S13
Silverman SL, Schousboe JT, Gold DT (2011) Oral bisphosphonate compliance and persistence: a matter of choice? Osteoporos Int 22:21–26
Hamilton B, McCoy K, Taggart H (2003) Tolerability and compliance with risedronate in clinical practice. Osteoporos Int 14:259–262
Tosteson AN, Grove MR, Hammond CS, Moncur MM, Ray GT, Hebert GM, Pressman AR, Ettinger B (2003) Early discontinuation of treatment for osteoporosis. Am J Med 115:209–216
Simon JA, Lewiecki EM, Smith ME, Petruschke RA, Wang L, Palmisano JJ (2002) Patient preference for once-weekly alendronate 70 mg versus once-daily alendronate 10 mg: a multicenter, randomized, open-label, crossover study. Clin Ther 24:1871–1886
Thiébaud D, Burckhardt P, Kriegbaum H, Huss H, Mulder H, Juttmann JR, Schöter KH (1997) Three monthly intravenous injections of ibandronate in the treatment of postmenopausal osteoporosis. Am J Med 103:298–307
Adami S, Felsenberg D, Christiansen C, Robinson J, Lorenc RS, Mahoney P, Coutant K, Schimmer RC, Delmas PD (2004) Efficacy and safety of ibandronate given by intravenous injection once every 3 months. Bone 34:881–889
Delmas PD, Adami S, Strugala C, Stakkestad JA, Reginster JY, Felsenberg D, Christiansen C, Civitelli R, Drezner MK, Recker RR, Bolognese M, Hughes C, Masanauskaite D, Ward P, Sambrook P, Reid DM (2006) Intravenous ibandronate injections in postmenopausal women with osteoporosis: one-year results from the dosing intravenous administration study. Arthritis Rheum 54:1838–1846
Eisman JA, Civitelli R, Adami S, Czerwinski E, Recknor C, Prince R, Reginster JY, Zaidi M, Felsenberg D, Hughes C, Mairon N, Masanauskaite D, Reid DM, Delmas PD, Recker RR (2008) Efficacy and tolerability of intravenous ibandronate injections in postmenopausal osteoporosis: 2-year results from the DIVA study. J Rheumatol 35:488–497
Bianchi G, Czerwinski E, Kenwright A, Burdeska A, Recker RR, Felsenberg D (2012) Long-term administration of quarterly IV ibandronate is effective and well tolerated in postmenopausal osteoporosis: 5-year data from the DIVA study long-term extension. Osteoporos Int 23:1769–1778
Feldstein AC, Weycker D, Nichols GA, Oster G, Rosales G, Boardman DL, Perrin N (2009) Effectiveness of bisphosphonate therapy in a community setting. Bone 44:153–159
Barrett-Connor E, Wade SW, Do TP, Satram-Hoang S, Stewart R, Gao G, Macarios D (2012) Treatment satisfaction and persistence among postmenopausal women on osteoporosis medications: 12-month results from POSSIBLE US™. Osteoporos Int 23:733–741
Hadji P, Minne H, Pfeifer M, Bourgeois P, Fardellone P, Licata A, Devas V, Masanauskaite D, Barrett-Connor E (2008) Treatment preference for monthly oral ibandronate and weekly oral alendronate in women with postmenopausal osteoporosis: a randomized, crossover study (BALTO II). Joint Bone Spine 75:303–310
Silverman S, Gold DT (2010) Compliance and persistence with osteoporosis medications: a critical review of the literature. Rev Endocr Metab Disord 11:275–280
Gold DT, Martin BC, Frytak JR, Amonkar MM, Cosman F (2007) A claims database analysis of persistence with alendronate therapy and fracture risk in post-menopausal women with osteoporosis. Curr Med Res Opin 23:585–594
Hadji P, Claus V, Ziller V, Intorcia M, Kostev K, Steinle T (2012) GRAND: the German retrospective cohort analysis on compliance and persistence and the associated risk of fractures in osteoporotic women treated with oral bisphosphonates. Osteoporos Int 23:223–231
Ziller V, Kostev K, Kyvernitakis I, Boeckhoff J, Hadji P (2012) Persistence and compliance of medications used in the treatment of osteoporosis-analysis using a large-scale, representative, longitudinal German database. Int J Clin Pharmacol Ther 50:315–322
Seeman E, Compston J, Adachi J, Brandi ML, Cooper C, Dawson-Hughes B, Jönsson B, Pols H, Cramer JA (2007) Non-compliance: the Achilles’ heel of anti-fracture efficacy. Osteoporos Int 18:711–719
Sarrel PM (1999) Improving adherence to hormone replacement therapy with effective patient-physician communication. Am J Obstet Gynecol 180:S337–S340
Bauer DC, Black D, Ensrud K, Thompson D, Hochberg M, Nevitt M, Musliner T, Freedholm D (2000) Upper gastrointestinal tract safety profile of alendronate: the fracture intervention trial. Arch Intern Med 160:517–525
Greenspan S, Field-Munves E, Tonino R, Smith M, Petruschke R, Wang L, Yates J, de Papp AE, Palmisano J (2002) Tolerability of once-weekly alendronate in patients with osteoporosis: a randomized, double-blind, placebo-controlled study. Mayo Clin Proc 77:1044–1052
Eisman JA, Rizzoli R, Roman-Ivorra J, Lipschitz S, Verbruggen N, Gaines KA, Melton ME (2004) Upper gastrointestinal and overall tolerability of alendronate once weekly in patients with osteoporosis: results of a randomized, double-blind, placebo-controlled study. Curr Med Res Opin 20:699–705
Dennison EM, Compston JE, Flahive J, Siris ES, Gehlbach SH, Adachi JD, Boonen S, Chapurlat R, Díez-Pérez A, Anderson FA Jr, Hooven FH, LaCroix AZ, Lindsay R, Netelenbos JC, Pfeilschifter J, Rossini M, Roux C, Saag KG, Sambrook P, Silverman S, Watts NB, Greenspan SL, Premaor M, Cooper C, Investigators GLOW (2012) Effect of co-morbidities on fracture risk: findings from the Global Longitudinal Study of Osteoporosis in Women (GLOW). Bone 50:1288–1293
Curtis JR, Xi J, Westfall AO, Cheng H, Lyles K, Saag KG, Delzell E (2009) Improving the prediction of medication compliance: the example of bisphosphonates for osteoporosis. Med Care 47:334–341
Breuil V, Cortet B, Cotté FE, Arnould B, Dias-Barbosa C, Gaudin AF, Regnault A, Roborel de Climens A, Legrand E (2012) Validation of the adherence evaluation of osteoporosis treatment (ADEOS) questionnaire for osteoporotic post-menopausal women. Osteoporos Int 23:445–455
Häussler B, Gothe H, Göl D, Glaeske G, Pientka L, Felsenberg D (2007) Epidemiology, treatment and costs of osteoporosis in Germany—the BoneEVA Study. Osteoporos Int 18:77–84
Huybrechts KF, Ishak KJ, Caro JJ (2006) Assessment of compliance with osteoporosis treatment and its consequences in a managed care population. Bone 38:922–928
Sunyecz JA, Mucha L, Baser O, Barr CE, Amonkar MM (2008) Impact of compliance and persistence with bisphosphonate therapy on health care costs and utilization. Osteoporos Int 19:1421–1429
Acknowledgments
This study was funded by Roche Pharma AG, Grenzach-Wyhlen, Germany. The authors thank Dr. A. Defer, Dr. J. Pfeilschifter, and Dr. O. Gröschel for their participation in the study. The authors also thank Dr. M. Hoque from ACUMED for providing medical writing support, funded by Roche.
Conflicts of interest
Peyman Hadji: has received honoraria and research funding from Amgen, Roche, GlaxoSmithKline, Novartis, MSD, Daiichi Sankyo, Pfizer, Procter & Gamble, Eli Lilly, and Nycomed.
Lorenz Hofbauer receives honoraria for lectures and advisory board meetings from Amgen, Merck, and Novartis.
Dieter Felsenberg receives payment for consultancy/advisory boards from Amgen, Chugai Pharmaceutical Co. Ltd., GlaxoSmithKline, Lilly, MSD, Novartis, Nycomed, Roche, Servier, and Teva Pharmaceutical Industries Ltd.; lecture fees from Amgen, Chugai, GE Healthcare, GlaxoSmithKline, Lilly, MSD, Novartis, Nycomed, Roche, Servier, Teva Pharmaceutical Industries Ltd., and Warner Chilcott; and grant support from Amgen, Chugai, Lilly, MSD, Novartis, Nycomed, Roche, Teva Pharmaceutical Industries Ltd., and Servier.
Andreas Kurth is a speaker for Roche and an active member on the advisory board for BonViva; is a speaker for Biomet, DFine Europe, Medtronic, Servier, Novartis, Lilly, Amgen, BMS, GlaxoSmithKline, Baxter, Boehringer Ingelheim, and Anwerina; and is a consultant for DFine Europe, Biomet, Servier, Amgen, Boehringer Ingelheim, and BMS.
Michael Amling is a consultant and speaker for Roche, Novartis, Procter and Gamble, MSD, Servier, and Lilly. No royalties were received.
Julia Annabel Kandenwein is an employee of Roche Pharma Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hadji, P., Felsenberg, D., Amling, M. et al. The non-interventional BonViva Intravenous Versus Alendronate (VIVA) study: real-world adherence and persistence to medication, efficacy, and safety, in patients with postmenopausal osteoporosis. Osteoporos Int 25, 339–347 (2014). https://doi.org/10.1007/s00198-013-2515-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-013-2515-2