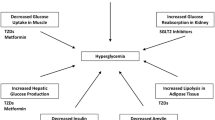
Abstract
Thiazolidinediones (TZDs) are agonists of the peroxisome proliferator-activated receptor gamma (PPARγ) nuclear transcription factor. Two members of this drug class, rosiglitazone and pioglitazone, are commonly used in the management of type II diabetes mellitus, and play emerging roles in the treatment of other clinical conditions characterized by insulin resistance. Over the past decade, a consistent body of in vitro and animal studies has demonstrated that PPARγ signaling regulates the fate of pluripotent mesenchymal cells, favoring adipogenesis over osteoblastogenesis. Treatment of rodents with TZDs decreases bone formation and bone mass. Until recently, there were no bone-related data available from studies of TZDs in humans. In the past year, however, several clinical studies have reported adverse skeletal actions of TZDs in humans. Collectively, these investigations have demonstrated that the TZDs currently in clinical use decrease bone formation and accelerate bone loss in healthy and insulin-resistant individuals, and increase the risk of fractures in the appendicular skeleton in women with type II diabetes mellitus. These observations should prompt clinicians to evaluate fracture risk in patients for whom TZD therapy is being considered, and initiate skeletal protection in at-risk individuals.


Similar content being viewed by others
References
Henney JE (2000) From the food and drug administration. JAMA 283:2228
Yki-Jarvinen H (2004) Thiazolidinediones. N Engl J Med 351:1106–1118
Yki-Jarvinen H (2005) The PROactive study: some answers, many questions. Lancet 366:1241–1242
Kahn SE, Haffner SM, Heise MA et al (2006) Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355:2427–2443
Stout DL, Fugate SE (2005) Thiazolidinediones for treatment of polycystic ovary syndrome. Pharmacotherapy 25:244–252
Dream Trial Investigators (2006) Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 368:1096–1105
Nissen SE, Wolski K (2007) Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356:2457–2471
Psaty BM, Furberg CD (2007) Rosiglitazone and cardiovascular risk. N Engl J Med 356:2522–2524
[no authors listed] (2007) Rosiglitazone: seeking a balanced perspective. Lancet 369:1834
Krall RL (2007) Cardiovascular safety of rosiglitazone. Lancet 369:1995–1996
Home PD, Pocock SJ, Beck-Nielsen H et al (2007) Rosiglitazone evaluated for cardiovascular outcomes—an interim analysis. N Engl J Med 357:28–38
Dormandy JA, Charbonnel B, Eckland DJA et al (2005) Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366:1279–1289
Vestergaard P (2007) Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos Int 18:427–444
Strotmeyer ES, Cauley JA, Schwartz AV et al (2005) Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: the Health, Aging, and Body Composition study. Arch Intern Med 165:1612–1617
Nicodemus KK, Folsom AR (2001) Type 1 and type 2 diabetes and incident hip fractures in postmenopausal women. Diabetes Care 24:1192–1197
Schwartz AV, Sellmeyer DE, Ensrud KE et al (2001) Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab 86:32–38
Bonds DE, Larson JC, Schwartz AV et al (2006) Risk of fracture in women with type 2 diabetes: the Women’s Health Initiative Observational Study. J Clin Endocrinol Metab 91:3404–3410
Vestergaard P, Rejnmark L, Mosekilde L (2005) Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia 48:1292–1299
Ivers RQ, Cumming RG, Mitchell P et al (2001) Diabetes and risk of fracture: the Blue Mountains Eye Study. Diabetes Care 24:1198–1203
Ahmed LA, Joakimsen RM, Berntsen GK et al (2006) Diabetes mellitus and the risk of non-vertebral fractures: the Tromso study. Osteoporos Int 17:495–500
Miao J, Brismar K, Nyren O et al (2005) Elevated hip fracture risk in type 1 diabetic patients: a population-based cohort study in Sweden. Diabetes Care 28:2850–2855
Whiteside GT, Boulet JM, Sellers R et al (2006) Neuropathy-induced osteopenia in rats is not due to a reduction in weight born on the affected limb. Bone 38:387–393
Cundy TF, Edmonds ME, Watkins PJ (1985) Osteopenia and metatarsal fractures in diabetic neuropathy. Diabet Med 2:461–464
Zhou Z, Immel D, Xi C-X et al (2006) Regulation of osteoclast function and bone mass by RAGE. J Exp Med 203:1067–1080
Katayama Y, Akatsu T, Yamamoto M et al (1996) Role of nonenzymatic glycosylation of type I collagen in diabetic osteopenia. J Bone Miner Res 11:931–937
Miyata T, Notoya K, Yoshida K et al (1997) Advanced glycation end products enhance osteoclast-induced bone resorption in cultured mouse unfractionated bone cells and in rats implanted subcutaneously with devitalized bone particles. J Am Soc Nephrol 8:260–270
Gimble JM, Robinson CE, Wu X et al (1996) Peroxisome proliferator-activated receptor-gamma activation by thiazolidinediones induces adipogenesis in bone marrow stromal cells. Mol Pharmacol 50:1087–1094
Johnson TE, Vogel R, Rutledge SJ et al (1999) Thiazolidinedione effects on glucocorticoid receptor-mediated gene transcription and differentiation in osteoblastic cells. Endocrinology 140:3245–3254
Jeon MJ, Kim JA, Kwon SH et al (2003) Activation of peroxisome proliferator-activated receptor-gamma inhibits the Runx2-mediated transcription of osteocalcin in osteoblasts. J Biol Chem 278:23270–23277
Jackson SM, Demer LL (2000) Peroxisome proliferator-activated receptor activators modulate the osteoblastic maturation of MC3T3-E1 preosteoblasts. FEBS Lett 471:119–124
Diascro DD, Vogel RL, Johnson TE et al (1998) High fatty acid content in rabbit serum is responsible for the differentiation of osteoblasts into adipocyte-like cells. J Bone Miner Res 13:96–106
Nuttall ME, Patton AJ, Olivera DL et al (1998) Human trabecular bone cells are able to express both osteoblastic and adipocytic phenotype: implications for osteopenic disorders. J Bone Miner Res 13:371–382
Mbalaviele G, Abu-Amer Y, Meng A et al (2000) Activation of peroxisome proliferator-activated receptor-gamma pathway inhibits osteoclast differentiation. J Biol Chem 275:14388–14393
Chan BY, Gartland A, Wilson PJM et al (2007) PPAR agonists modulate human osteoclast formation and activity in vitro. Bone 40:149–159
Lecka-Czernik B, Gubrij I, Moerman EJ et al (1999) Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARgamma2. J Cell Biochem 74:357–371
Khan E, Abu-Amer Y (2003) Activation of peroxisome proliferator-activated receptor-gamma inhibits differentiation of preosteoblasts. J Lab Clin Med 142:29–34
Kawaguchi H, Akune T, Yamaguchi M et al (2005) Distinct effects of PPARgamma insufficiency on bone marrow cells, osteoblasts, and osteoclastic cells. J Bone Miner Metab 23:275–279
Lecka-Czernik B, Moerman EJ, Grant DF et al (2002) Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology 143:2376–2384
Kim SH, Yoo CI, Kim HT et al (2006) Activation of peroxisome proliferator-activated receptor-gamma (PPARgamma) induces cell death through MAPK-dependent mechanism in osteoblastic cells. Toxicol Appl Pharmacol 215:198–207
Okazaki R, Toriumi M, Fukumoto S et al (1999) Thiazolidinediones inhibit osteoclast-like cell formation and bone resorption in vitro. Endocrinology 140:5060–5065
Reid IR, Cornish J, Baldock PA (2006) Nutrition-related peptides and bone homeostasis. J Bone Miner Res 21:495–500
Fain JN, Cowan GS Jr, Buffington C et al (2000) Regulation of leptin release by troglitazone in human adipose tissue. Metabolism 49:1485–1490
Williams LB, Fawcett RL, Waechter AS et al (2000) Leptin production in adipocytes from morbidly obese subjects: stimulation by dexamethasone, inhibition with troglitazone, and influence of gender. J Clin Endocrinol Metab 85:2678–2684
Cornish J, Callon KE, Bava U et al (2002) Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol 175:405–415
Ducy P, Amling M, Takeda S et al (2000) Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100:197–207
Maeda N, Takahashi M, Funahashi T et al (2001) PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 50:2094
Oshima K, Nampei A, Matsuda M et al (2005) Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun 331:520–526
Williams GA, Callon KE, Watson M et al (2006) Adiponectin knock-out mice have increased trabecular number and bone volume at 14 weeks of age. Australia New Zealand Bone and Mineral Society, Port Douglas, QA, O28
Cornish J, Callon KE, Reid IR (1996) Insulin increases histomorphometric indices of bone formation in vivo. Calcif Tissue Int 59:492–495
Cornish J, Callon KE, King AR et al (1998) Systemic administration of amylin increases bone mass, linear growth, and adiposity in adult male mice. Am J Physiol 38:E694–E699
Cornish J, Callon KE, Bava U et al (2007) Preptin, another peptide product of the pancreatic beta-cell, is osteogenic in vitro and in vivo. Am J Physiol Endocrinol Metab 292:E117–E122
Lecka-Czernik B, Ackert-Bicknell C, Adamo ML et al (2007) Activation of peroxisome proliferator-activated receptor gamma (PPARgamma) by rosiglitazone suppresses components of the insulin-like growth factor regulatory system in vitro and in vivo. Endocrinology 148:903–911
Jennermann C, Triantafillou J, Cowan D et al (1995) Effects of thiazolidinediones on bone turnover in the rat. J Bone Miner Res 10 [Suppl 1]:S241
Rzonca SO, Suva LJ, Gaddy D et al (2004) Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology 145:401–406
Ali AA, Weinstein RS, Stewart SA et al (2005) Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology 146:1226–1235
Soroceanu MA, Miao D, Bai X-Y et al (2004) Rosiglitazone impacts negatively on bone by promoting osteoblast/osteocyte apoptosis. J Endocrinol 183:203–216
Li M, Pan LC, Simmons HA et al (2006) Surface-specific effects of a PPAR-gamma agonist, darglitazone, on bone in mice. Bone 39:796–806
Lazarenko OP, Rzonca SO, Suva LJ et al (2006) Netoglitazone is a PPAR-gamma ligand with selective effects on bone and fat. Bone 38:74–84
Tornvig L, Mosekilde LI, Justesen J et al (2001) Troglitazone treatment increases bone marrow adipose tissue volume but does not affect trabecular bone volume in mice. Calcif Tissue Int 69:46–50
Sottile V, Seuwen K, Kneissel M (2004) Enhanced marrow adipogenesis and bone resorption in estrogen-deprived rats treated with the PPARgamma agonist BRL49653 (rosiglitazone). Calcif Tissue Int 75:329–337
Lazarenko OP, Rzonca SO, Hogue WR et al (2007) Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology 148:2669–2680
Akune T, Ohba S, Kamekura S et al (2004) PPAR-gamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest 113:846–855
Klein RF, Allard J, Avnur Z et al (2004) Regulation of bone mass in mice by the lipoxygenase gene Alox15. Science 303:229–232
Okazaki R, Miura M, Toriumi M et al (1999) Short-term treatment with troglitazone decreases bone turnover in patients with type 2 diabetes mellitus. Endocr J 46:795–801
Watanabe S, Takeuchi Y, Fukumoto S et al (2003) Decrease in serum leptin by troglitazone is associated with preventing bone loss in type 2 diabetic patients. J Bone Miner Metab 21:166–171
Schwartz AV, Sellmeyer DE, Vittinghoff E et al (2006) Thiazolidinedione use and bone loss in older diabetic adults. J Clin Endocrinol Metab 91:3349–3354
Yaturu S, Bryant B, Jain SK (2007) Thiazolidinediones treatment decreases bone mineral density in type 2 diabetic men. Diabetes Care 30:1574–1576
Grey A, Bolland M, Gamble G et al (2007) The peroxisome proliferator-activated receptor-gamma agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab 92:1305–1310
Berberoglu Z, Gursoy A, Bayraktar N et al (2007) Rosiglitazone decreases serum bone-specific alkaline phosphatase activity in postmenopausal diabetic women. J Clin Endocrinol Metab 92:3523–3530
Ton FN, Gunawardene SC, Lee H et al (2005) Effects of low-dose prednisone on bone metabolism. J Bone Miner Res 20:464–470
Sanders KM, Seeman E, Ugoni AM et al (1999) Age- and gender-specific rate of fractures in Australia: a population-based study. Osteoporos Int 10:240–247
Garraway WM, Stauffer RN, Kurland LT et al (1979) Limb fractures in a defined population. I. Frequency and distribution. Mayo Clin Proc 54:701–707
US Food and Drug Administration. Accessed at http://www.fda.gov/medwatch/safety/2007/Avandia_GSK_Ltr.pdf on 3 March 2007
US Food and Drug Administration. Accessed at http://www.fda.gov/medwatch/safety/2007/Actosmar0807.pdf on 13 March 2007
Saag KG, Emkey R, Schnitzer TJ et al (1998) Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Induced Osteoporosis Intervention Study Group. N Engl J Med 339:292–299
Adachi JD, Saag KG, Delmas PD et al (2001) Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum 44:202–211
Grey AB, Cundy TF, Reid IR (1994) Continuous combined oestrogen/progestin therapy is well tolerated and increases bone density at the hip and spine in post-menopausal osteoporosis. Clin Endocrinol (Oxf) 40:671–677
National Osteoporosis Foundation (2003) Physician’s guide to prevention and treatment of osteoporosis. In. National Osteoporosis Foundation, Washington, DC
American Association of Clinical Endocrinologists (2003) Medical guidelines for clinical practice for the prevention and treatment of postmenopausal osteoporosis. Endocr Pract 9:544–564
Acknowledgements
Funding support is provided by the Health Research Council of New Zealand. The author is grateful to Ian Reid MD for reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grey, A. Skeletal consequences of thiazolidinedione therapy. Osteoporos Int 19, 129–137 (2008). https://doi.org/10.1007/s00198-007-0477-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-007-0477-y