Abstract
Purpose
Single studies of Noninvasive Ventilation (NIV) in the management of acute respiratory failure in chest trauma patients have produced controversial findings. The aim of this study is to critically review the literature to investigate whether NIV reduces mortality, intubation rate, length of stay and complications in patients with chest trauma, compared to standard therapy.
Methods
We performed a systematic review and meta-analysis of randomized controlled trials, prospective and retrospective observational studies, by searching PubMed, EMBASE and bibliographies of articles retrieved. We screened for relevance studies that enrolled adults with chest trauma who developed mild to severe acute respiratory failure and were treated with NIV. We included studies reporting at least one clinical outcome of interest to perform a meta-analysis.
Results
Ten studies (368 patients) met the inclusion criteria and were included for the meta-analysis. Five studies (219 patients) reported mortality and results were quite homogeneous across studies, with a summary relative risk for patients treated with NIV compared with standard care (oxygen therapy and invasive mechanical ventilation) of 0.26 (95 % confidence interval 0.09–0.71, p = 0.003). There was no advantage in mortality of continuous positive airway pressure over noninvasive pressure support ventilation. NIV significantly increased arterial oxygenation and was associated with a significant reduction in intubation rate, in the incidence of overall complications and infections.
Conclusions
These results suggest that NIV could be useful in the management of acute respiratory failure due to chest trauma.
Similar content being viewed by others
Introduction
Chest trauma is reported to occur in 20 % of multiple trauma patients [1] and accounts for 20–40 % of all trauma deaths, second only to head and spinal injuries. Elderly patients and patients with poor respiratory function are the most likely to develop acute respiratory failure [1–3]. The most common injuries associated with chest trauma are rib fractures, pneumothorax, hemothorax, flail chest and pulmonary contusion [2, 4–6]. Lung function is impaired after chest trauma primarily by direct parenchymal damage resulting in hemorrhage and lung disruption, and subsequently, by the activation of systemic inflammation that causes alveolar capillary barrier dysfunction and increases extravascular lung water. All these pathophysiologic events in addition to excessive bronchial secretions and airway colonization can promote alveolar collapse, ventilation perfusion mismatch, lung consolidation and lung infection, causing mild hypoxemia to severe acute respiratory distress syndrome (ARDS). The incidence of post-traumatic ARDS is between 8 and 37 % of trauma patients [7–11] with the highest percentage within 3 days after the traumatic event [9] and the associated mortality ranging from 16 to 29 % [7, 8, 12]. Patients who developed ARDS had a significantly higher mortality rate [8, 13], longer hospital and intensive care length of stay and higher hospital cost than trauma patients without ARDS [12].
Clinical management of less severely injured patients generally involves only supplemental oxygen therapy, aggressive chest physiotherapy and pain relief, without any ventilator support [14–16]. In 40–60 % of patients, however, cardiovascular failure, flail chest, pulmonary contusion and the need for major surgery are risk factors for intubation and mechanical ventilation [17, 18]. However, invasive mechanical ventilation lasting more than 5 days increases the risk of late-onset pneumonia and bacteremia, which in turn rises morbidity and mortality [19, 20]. Noninvasive ventilation (NIV) reduced the need for intubation and improved the outcome in selected patients with acute cardiogenic pulmonary edema [21, 22], exacerbation of chronic obstructive pulmonary disease (COPD) [23], and hypoxemic respiratory failure [24]. NIV also significantly lowered the incidence of ventilator-associated pneumonia [25, 26] and the requirement for sedatives, with better patient comfort compared to invasive mechanical ventilation [27].
Since the first report by Uretzky et al. [28] on the use of NIV in chest trauma patients, several randomized controlled trials, prospective observational studies and case reports have described the application of NIV in trauma [27]. In a multicenter survey of post-traumatic patients with acute respiratory failure the rate of treatment failures with NIV was 18 %, similar to those observed in patients with cardiogenic pulmonary edema [20]. However, the British Thoracic Society Guidelines suggested, with a low grade of recommendation, the use of NIV in patients with chest trauma who remain hypoxic despite adequate regional anesthesia and high oxygen flow [29]. Thus, irrespective of the widespread use of NIV and its unique benefits compared to mechanical ventilation, there is no clear consensus on its use in chest trauma [27].
These considerations led us to conduct a systematic review and a meta-analysis to investigate whether NIV in the form of either continuous positive airway pressure (CPAP), bi-level positive airway pressure (BiPAP) or noninvasive pressure support ventilation (NIPSV) reduced mortality, intubation rate, length of stay and complications compared to standard medical therapy (oxygen therapy or invasive mechanical ventilation).
Materials and methods
Search strategy
We performed a computerized search of MEDLINE/PubMed and EMBASE. All databases were searched from inception until July 2012. Our search was limited to studies on humans and those published in English. We used the following search keywords and terms: (chest trauma OR abdominal trauma OR pulmonary contusion OR posttraumatic hypoxemic respiratory failure OR trauma patients OR pneumothorax OR rib fractures) AND (noninvasive ventilation OR NIV OR continuous positive airway pressure OR CPAP). Three reviewers (DC, SC, SF) screened citation titles and abstracts independently. We reviewed the references of all articles retrieved and reviewed articles to identify additional potentially eligible studies. In case of disagreement the authors reviewed the article in question together until they reached a consensus. We identified and deleted any duplicate papers. All potentially eligible papers were retrieved in full.
Data were collected in a datasheet including the following: publication (first author’s name, year, journal), study design, number of patients enrolled and outcomes.
Study selection
We screened for relevance studies that enrolled adults with chest trauma who developed mild to severe acute respiratory failure, were admitted to a trauma service, emergency department or intensive care unit (ICU) and were treated with NIV strategy.
Then we made a quantitative synthesis performing a meta-analysis. For this purpose we selected the following study designs: randomized controlled trials (RCTs), prospective observational trials (before and after treatment) and retrospective observational studies.
NIV was applied as CPAP or BiPAP (noninvasive intermittent positive pressure ventilation with two different levels of positive pressure) or NIPSV (noninvasive intermittent pressure support ventilation).
We considered the use of CPAP or BiPAP or NIPSV delivered using any interface as experimental strategy, compared to mechanical ventilation or unassisted supplemental oxygen therapy.
We included studies reporting at least one of the following clinical outcomes: gas exchange, respiratory rate, length of stay in ICU or hospital, complications, intubation rate, infections and mortality.
Statistical analysis
We ran our meta-analysis including all studies (observational and RCTs). For continuous outcomes (gas exchange, respiratory rate, length of stay in ICU or hospital), we extracted the mean responses, standard deviations (SD), and the group sizes, then we calculated the DerSimonian and Laird random effect mean weighted difference (WMD) using the pooled SDs of the treatment and control groups [30]. When the mean and SDs were not available we calculated the SD with the formula: SD = (max – min)/(2 × 1.96) [31]. (We could have used a larger divisor, e.g. 6, but we preferred a more conservative approach). Some were before-after studies, but since we did not have access to individual (matched) values, we could not calculate the correct SD of the mean difference. Therefore we considered them (conservatively) as unmatched studies.
For dichotomous outcomes (adverse events including infections, intubation rate, complications and mortality) we calculated summary relative risk (RR) estimates using DerSimonian and Laird random—effect models [30]. When a study contained a zero cell we added 0.5 to each cell to obtain finite variance estimators. The heterogeneity across studies was measured by the I2 statistic [32]. I2 values of 25–50 % indicate moderate inconsistency, whereas larger values reflect large inconsistencies among studies. We evaluated the possibility of publication bias using funnel plots and Begg’s and Egger’s test [30]. Statistical analyses were performed with Stata 12 (StataCorp. 2011. Stata: Release 12. Statistical Software. College Station, TX: StataCorp LP).
Results
Search and study selection
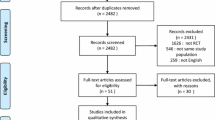
We identified 263 articles using the search strategy. The selection process is shown in Fig. 1.
After screening titles and abstracts for relevance and compliance with our inclusion criteria we excluded 224 articles that were considered as not relevant or that involved enrolling newborn and pediatric patients (Fig. 1) We added one other study from the reference list of the articles retrieved.
After examination of the full text of the selected papers we included 10 studies (observational studies and RCTs) for the meta-analysis (Tables 1 and 2). These studies enrolled patients admitted to ICUs and emergency departments in different countries: Italy [33, 34], USA [42], Spain [35, 36], Greece [37], South Africa [38, 39], Turkey [40] and Australia [41].
The total number of patients was 368. Selection criteria for the quantitative synthesis are presented in Fig. 1. Seven studies enrolled chest trauma patients with acute respiratory failure. In the majority the criteria for acute respiratory failure were a combination of impairment of oxygenation and increase in respiratory rate.
All studies used a face mask. Noninvasive positive pressure was delivered as CPAP [38–43], BiPAP [35–37, 41, 43] and as noninvasive pressure support ventilation (NIPSV) [33, 34, 37, 41, 43].
Effect of NIV on mortality
Five studies (219 patients) reported information on mortality. The pooled results are shown in Fig. 2. There were 3/101 (3.0 %) deaths in the NIV group compared to 27/118 (22.9 %) in the control group. There was no heterogeneity across studies.
Effect of Noninvasive Ventilation (NIV) on mortality in chest trauma patients. Treatment group represents the use of NIV. Control group represents mechanical ventilation or unassisted supplemental oxygen therapy. Vertical solid line null effect, Vertical dotted line overall mortality effect of treatment, Boxes and horizontal lines relative risk (RR) and 95% confidence interval, CI confidence interval, NIV noninvasive ventilation
The estimate of the RR for mortality in patients treated with NIV compared with standard care was 0.26 (95 % confidence interval 0.09 to 0.71, p = 0.003).
Effect of NIV on intubation rate, complications and length of stay
NIV significantly reduced the intubation rate [RR 0.32 (95 % confidence interval 0.12 to 0.86, p = 0.023)], overall complications and infection [RR 0.37 (95 % confidence interval 0.24 to 0.57, p < 0.001) and RR 0.34 (95 % confidence interval 0.20 to 0.58, p < 0.001), respectively] (Table 3).
The length of ICU stay was also significantly shorter: WMD −2.4 (95 % confidence interval −3.9 to −1.0, p = 0.001). Hospital length of stay was shorter, but without reaching significance: WMD −4.0 (95 % confidence interval −9.7 to 1.6, p = 0.162), although significant heterogeneity existed among studies (I2 = 94.9 %) (Table 3).
Effect of NIV on gas exchange and respiratory rate
NIV increased the arterial oxygenation WMD of 101.0 (95 % confidence interval 10.6 to 191.5, p = 0.029), but did not affect the arterial carbon dioxide WMD of −1.03 (95 % confidence interval −5.19 to 3.13, p = 0.627) (Fig. 3, upper and middle panel). The respiratory rate was lower in the NIV group WMD of −9.25 (95 % confidence interval −15.72 to −2.79, p = 0.005) (Fig. 3, lower panel).
Effect of Noninvasive Ventilation on respiratory parameters in chest trauma patients. Upper panel oxygenation (P/F)—ratio of arterial oxygen concentration to the fraction of inspired oxygen, Middle panel PaCO2, Lower panel respiratory rate. Treatment group represents the use of NIV. Control group represents mechanical ventilation or unassisted supplemental oxygen therapy. Vertical solid line null effect, Vertical dotted line overall effect of treatment, Boxes and horizontal lines weighted mean difference (WMD) and 95% confidence interval, CI confidence interval, SD standard deviation, NIV noninvasive ventilation, N number of patients
Discussion
To our knowledge this is the first systematic review and meta-analysis of the effect of NIV on chest trauma patients. We found that NIV significantly reduced mortality, requirement for tracheal intubation, length of ICU stay and improved oxygenation.
In severe chest trauma patients, a lung lesion (i.e. pulmonary contusion, pneumothorax) and/or thoracic injury (hemothorax, rib fractures, flail chest) with inadequate pain relief and systemic inflammatory activation can promote acute respiratory failure due to alveolar collapse and impaired alveolar fluid clearance [44]. Therefore for several decades now, invasive mechanical ventilation with positive end-expiratory pressure (PEEP) has been suggested as the only possible ventilator support to improve gas exchange [45, 46]. Invasive mechanical ventilation has been used in up to 50 % of chest trauma patients [17, 18]. Although tracheal intubation and mechanical ventilation are life-saving tools, they can induce barotrauma, ventilator-associated infections and other complications relating to sedation and immobility [27].
NIV promotes a wider and earlier use of mechanical ventilation outside the ICU [27, 47–50], thus limiting “lung collapse” and the risk of early-onset pneumonia [51]. Several trials and meta-analysis indicate a beneficial effect of NIV on outcome in patients with acute cardiogenic pulmonary edema and exacerbations of COPD [23, 52]. Although some studies reported that NIV reduced mortality in patients with ARDS and severe community-acquired pneumonia [22, 24], its use is still debated [53, 54].
The mortality rate in ARDS due to trauma is lower than for ARDS due to pneumonia or sepsis [10, 11], although the development of ARDS per se increases mortality. NIV may significantly lower the risk of ARDS developing, because of its role in lung recruitment (i.e. limiting lung collapse) and/or in preventing lung infections [27, 51]. Among the studies we analyzed only Hernandez et al. [36] did not find a lower mortality rate. Interestingly, NIV’s effects on mortality were not related to the treatment modality. CPAP provides constant positive pressure throughout the respiratory cycle, while BiPAP or NIPSV provides intermittent positive pressure during inspiration, above the PEEP level. Theoretically BiPAP and NIPSV are more able to reduce the respiratory work of breathing and respiratory rates [55]. However, PEEP seems to influence the patient’s final outcome more than intermittent positive pressure.
In trauma patients the risk for intubation depends not only on the severity of gas exchange impairment, but also on the severity of trauma [14, 56], on the extension of lung contusion and on the hemodynamic status [56]. Early use of NIV, by favoring chest stabilization and lung recruitment, might prevent the failure of spontaneous breathing and avoid the need for intubation. In a prospective multicenter cohort study of hypoxemic patients after pulmonary contusion, the intubation rate was 18 %, significantly lower than in patients with community-acquired pneumonia or ARDS [20]. The risk of failure with NIV was associated with a higher SAPS II score and with persistent hypoxemia after 1 h of NIV [20]. Peter et al. [57] reported in a meta-analysis that NIV was associated with a significant reduction in the need for mechanical ventilation in all studies. However, the fact that no trauma patients were enrolled precluded any analysis in this subgroup.
The present meta-analysis found a significant reduction in the intubation rate with NIV (mean failure 14.5 %) and in the ICU length of stay as well as a lower infection rate. All except two of the studies [35, 42] were performed in ICUs, limiting the risk of bias from different locations, timing and nursing assistance [47].
The incidence of nosocomial pneumonia because of differences in the definition of pneumonia, patients’ characteristics and risk factors, was not homogeneous, ranging from 20 to 40 % [15, 19, 20, 58, 59]. Prolonged ventilation (>24 h) and PEEP significantly raised the risk of nosocomial pneumonia [59]. In a clinical survey, Nourdine et al. [26] reported that the incidence of nosocomial pneumonia and ventilator-associated pneumonia (VAP) was respectively 14.2 versus 30.3 and 4.4 vs 13.2 per 1,000 patients—days when noninvasive positive pressure ventilation was compared to endotracheal intubation. Among the cofactors related to nosocomial infection and VAP, trauma significantly raised the risk of infection compared to medical or surgical reasons for hospital admission [26]. In the present meta-analysis, NIV had a beneficial effect in reducing the infection rate and ICU length of stay.
After a direct or indirect lung insult, chest trauma patients can develop increased lung permeability and parenchymal collapse with ventilation-perfusion mismatch. NIV, by raising transpulmonary pressure, should recruit the collapsed and poorly ventilated lung regions, both reducing the work of breathing and ameliorating gas exchange. Like previous meta-analysis which found that NIV improved gas exchange in acute cardiogenic pulmonary edema, acute exacerbation of COPD and acute respiratory failure [23, 24, 60], we also found that NIV significantly ameliorated gas exchange in trauma patients. We found evidence of oxygenation improvement also when we restricted the meta-analysis to three studies [34, 37, 40] focusing on isolated chest trauma patients: WMD was 52.6 (versus 101.0 when considering all studies). For the other continuous outcomes the effect estimates (WMD or RR) were similar to those calculated in all the studies. Finally, binary outcomes were only evaluated among patients with isolated chest trauma.
Lung contusions are frequently associated with pneumothorax so any positive pressure ventilation might increase the entity of pneumothorax and eventually lead to hypertensive pneumothorax. In our analysis NIV use was not associated with any significant increase in the incidence of pneumothorax. Because CPAP involves a lower physiological risk of barotrauma with no less effect than NIPSV, it should be used as first-line treatment in patients with severe chest trauma [29].
The present meta-analysis has several limitations. First, the criteria for use of NIV in chest trauma differed among studies. Second, NIPSV—BiPAP pressure and ventilator characteristics were not homogeneous [47]. Third it was not possible to separate the effects of CPAP, BiPAP and NIPSV because of the paucity of studies for each group and moreover in one case these modalities were indifferently applied. Fourth, the number of eligible studies was relatively small. However, we found some possible evidence of publication bias only for length of hospital stay [36, 38, 40], for which we found p = 0.30 from Begg’s and p = 0.03 from Egger’s test, while for all other outcomes p values from either tests were always >0.40. We found high heterogeneity of effect across studies for the following continuous variables: oxygenation, PaCO2, respiratory rate, length of hospital stay. For these analyses, we tried to explain heterogeneity by stratifying the studies by potentially important variables (type of study, type of comparison, site of trauma, severity of hypoxemia, patients’ age, length of NIV treatment). However, due to the small number of studies, analysis was often not feasible or yielded unreliable results. For length of ICU stay, heterogeneity was mild (I2: 53.3 %, p = 0.09). For binary outcomes (complications, infections, intubation rate, mortality), there was a remarkable homogeneity of effect across studies (Table 3 and Fig. 2), with I2 = 0 and p values always >0.60.
Conclusion
This systematic review and meta-analysis indicates that the early use of NIV in chest trauma patients, facilitating stabilization of the chest and promoting the recruitment of collapsed lung regions, significantly reduces mortality and the intubation rate without increasing complications. However, NIV must be integrated with other medical and surgical clinical therapies.
References
Pinilla JC (1982) Acute respiratory failure in severe blunt chest trauma. J Trauma 22:221–226
Clark GC, Schecter WP, Trunkey DD (1988) Variables affecting outcome in blunt chest trauma: flail chest vs. pulmonary contusion. J Trauma 28:298–304
LoCicero J III, Mattox KL (1989) Epidemiology of chest trauma. Surg Clin North Am 69:15–19
Shorr RM, Crittenden M, Indeck M et al (1987) Blunt thoracic trauma. Analysis of 515 patients. Ann Surg 206:200–205
Wanek S, Mayberry JC (2004) Blunt thoracic trauma: flail chest, pulmonary contusion, and blast injury. Crit Care Clin 20:71–81
Ziegler DW, Agarwal NN (1994) The morbidity and mortality of rib fractures. J Trauma 37:975–979
Becher RD, Colonna AL, Enniss TM et al (2012) An innovative approach to predict the development of adult respiratory distress syndrome in patients with blunt trauma. J Trauma Acute Care Surg 73:1229–1235
Martin M, Salim A, Murray J et al (2005) The decreasing incidence and mortality of acute respiratory distress syndrome after injury: a 5-year observational study. J Trauma 59:1107–1113
Hudson LD, Milberg JA, Anardi D et al (1995) Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med 151:293–301
Navarrete-Navarro P, Rodriguez A, Reynolds N et al (2001) Acute respiratory distress syndrome among trauma patients: trends in ICU mortality, risk factors, complications and resource utilization. Intensive Care Med 27:1133–1140
Treggiari MM, Hudson LD, Martin DP et al (2004) Effect of acute lung injury and acute respiratory distress syndrome on outcome in critically ill trauma patients. Crit Care Med 32:327–331
Salim A, Martin M, Constantinou C et al (2006) Acute respiratory distress syndrome in the trauma intensive care unit: morbid but not mortal. Arch Surg 141:655–658
DuBose JJ, Putty B, Teixeira PG et al (2011) The relationship between post-traumatic ventilator-associated pneumonia outcomes and American College of Surgeons trauma centre designation. Injury 42:40–43
Karmakar MK, Ho AM (2003) Acute pain management of patients with multiple fractured ribs. J Trauma 54:615–625
Trinkle JK, Richardson JD, Franz JL et al (1975) Management of flail chest without mechanical ventilation. Ann Thorac Surg 19:355–363
Rico FR, Cheng JD, Gestring ML et al (2007) Mechanical ventilation strategies in massive chest trauma. Crit Care Clin 23:299–315 xi
Johnson JA, Cogbill TH, Winga ER (1986) Determinants of outcome after pulmonary contusion. J Trauma 26:695–697
Richardson JD, Adams L, Flint LM (1982) Selective management of flail chest and pulmonary contusion. Ann Surg 196:481–487
Antonelli M, Conti G, Riccioni L et al (1996) Noninvasive positive-pressure ventilation via face mask during bronchoscopy with BAL in high-risk hypoxemic patients. Chest 110:724–728
Antonelli M, Conti G, Moro ML et al (2001) Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: a multi-center study. Intensive Care Med 27:1718–1728
Mariani J, Macchia A, Belziti C et al (2011) Noninvasive ventilation in acute cardiogenic pulmonary edema: a meta-analysis of randomized controlled trials. J Card Fail 17:850–859
Peter JV, Moran JL, Phillips-Hughes J et al (2006) Effect of non-invasive positive pressure ventilation (NIPPV) on mortality in patients with acute cardiogenic pulmonary oedema: a meta-analysis. Lancet 367:1155–1163
Lightowler JV, Wedzicha JA, Elliott MW et al (2003) Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: cochrane systematic review and meta-analysis. BMJ 326:185
Keenan SP, Sinuff T, Cook DJ et al (2004) Does noninvasive positive pressure ventilation improve outcome in acute hypoxemic respiratory failure? A systematic review. Crit Care Med 32:2516–2523
Antonelli M, Conti G, Rocco M et al (1998) A comparison of noninvasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl J Med 339:429–435
Nourdine K, Combes P, Carton MJ et al (1999) Does noninvasive ventilation reduce the ICU nosocomial infection risk? A prospective clinical survey. Intensive Care Med 25:567–573
Nava S, Hill N (2009) Non-invasive ventilation in acute respiratory failure. Lancet 374:250–259
Uretzky G, Cotev S (1980) The use of continuous positive airway pressure in blast injury of the chest. Crit Care Med 8:486–489
BTS Standards of Care Committee (2002) Non-invasive ventilation in acute respiratory failure. Thorax 57:192–211
Egger M, Smith GD, Altman D (2001) Systematic reviews in health care: meta-analysis in context. BMJ Publishing Group, London
Rothman KJ, Greenland S (2008) Modern epidemiology, 3rd edn. Lippincott Williams & Wilkins, Philadelphia
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Beltrame F, Lucangelo U, Gregori D et al (1999) Noninvasive positive pressure ventilation in trauma patients with acute respiratory failure. Monaldi Arch Chest Dis 54:109–114
Gregoretti C, Beltrame F, Lucangelo U et al (1998) Physiologic evaluation of non-invasive pressure support ventilation in trauma patients with acute respiratory failure. Intensive Care Med 24:785–790
Ferrer M, Esquinas A, Leon M et al (2003) Noninvasive ventilation in severe hypoxemic respiratory failure: a randomized clinical trial. Am J Respir Crit Care Med 168:1438–1444
Hernandez G, Fernandez R, Lopez-Reina P et al (2010) Noninvasive ventilation reduces intubation in chest trauma-related hypoxemia: a randomized clinical trial. Chest 137:74–80
Xirouchaki N, Kondoudaki E, Anastasaki M et al (2005) Noninvasive bilevel positive pressure ventilation in patients with blunt thoracic trauma. Respiration 72:517–522
Bolliger CT, Van Eeden SF (1990) Treatment of multiple rib fractures. Randomized controlled trial comparing ventilatory with nonventilatory management. Chest 97:943–948
Linton DM, Potgieter PD (1982) Conservative management of blunt chest trauma. S Afr Med J 61:917–919
Gunduz M, Unlugenc H, Ozalevli M et al (2005) A comparative study of continuous positive airway pressure (CPAP) and intermittent positive pressure ventilation (IPPV) in patients with flail chest. Emerg Med J 22:325–329
Vidhani K, Kause J, Parr M (2002) Should we follow ATLS guidelines for the management of traumatic pulmonary contusion: the role of non-invasive ventilatory support. Resuscitation 52:265–268
Hurst JM, DeHaven CB, Branson RD (1985) Use of CPAP mask as the sole mode of ventilatory support in trauma patients with mild to moderate respiratory insufficiency. J Trauma 25:1065–1068
Munshi IA, DeHaven B, Kirton O et al (1999) Reengineering respiratory support following extubation: avoidance of critical care unit costs. Chest 116:1025–1028
Papadakos PJ, Karcz M, Lachmann B (2010) Mechanical ventilation in trauma. Curr Opin Anaesthesiol 23:228–232
Christensson P, Gisselsson L, Lecerof H et al (1979) Early and late results of controlled ventilation in flail chest. Chest 75:456–460
Diethelm AG, Battle W (1971) Management of flail chest injury: a review of 75 cases. Am Surg 37:667–670
Chiumello D, Esquinas AM, Moerer O et al (2012) A systematic technical review of the systems for the continuous positive airway pressure. Minerva Anestesiol 78:1385–1393
Sferrazza Papa GF, Di Marco F, Akoumianaki E et al (2012) Recent advances in interfaces for non-invasive ventilation: from bench studies to practical issues. Minerva Anestesiol 78:1146–1153
Pisani L, Carlucci A, Nava S (2012) Interfaces for noninvasive mechanical ventilation: technical aspects and efficiency. Minerva Anestesiol 78:1154–1161
Jaber S, Jung B (2011) Postoperative non-invasive ventilation outside the ICU: do not go too far! Minerva Anestesiol 77:9–10
Antonelli M, Moro ML, Capelli O et al (1994) Risk factors for early onset pneumonia in trauma patients. Chest 105:224–228
Masip J, Roque M, Sanchez B et al (2005) Noninvasive ventilation in acute cardiogenic pulmonary edema: systematic review and meta-analysis. JAMA 294:3124–3130
Agarwal R, Aggarwal AN, Gupta D (2010) Role of noninvasive ventilation in acute lung injury/acute respiratory distress syndrome: a proportion meta-analysis. Respir Care 55:1653–1660
Nava S, Schreiber A, Domenighetti G (2011) Noninvasive ventilation for patients with acute lung injury or acute respiratory distress syndrome. Respir Care 56:1583–1588
Chiumello D, Pelosi P, Carlesso E et al (2003) Noninvasive positive pressure ventilation delivered by helmet vs. standard face mask. Intensive Care Med 29:1671–1679
Tyburski JG, Collinge JD, Wilson RF et al (1999) Pulmonary contusions: quantifying the lesions on chest X-ray films and the factors affecting prognosis. J Trauma 46:833–838
Peter JV, Moran JL, Phillips-Hughes J et al (2002) Noninvasive ventilation in acute respiratory failure—a meta-analysis update. Crit Care Med 30:555–562
Joshi N, Localio AR, Hamory BH (1992) A predictive risk index for nosocomial pneumonia in the intensive care unit. Am J Med 93:135–142
Tejada AA, Bello DS, Chacon VE et al (2001) Risk factors for nosocomial pneumonia in critically ill trauma patients. Crit Care Med 29:304–309
Agarwal R, Aggarwal AN, Gupta D et al (2005) Non-invasive ventilation in acute cardiogenic pulmonary oedema. Postgrad Med J 81:637–643
Conflicts of interest
Dr. Gregoretti received fees for lectures from Vivisol, SapoLife and Covidien. He also received fees for consultancies from Covidien and Smith Medical.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chiumello, D., Coppola, S., Froio, S. et al. Noninvasive ventilation in chest trauma: systematic review and meta-analysis. Intensive Care Med 39, 1171–1180 (2013). https://doi.org/10.1007/s00134-013-2901-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-013-2901-4