Abstract
Purpose
Gut overgrowth is the pathophysiological event in the critically ill requiring intensive care. In relation to the risk of developing a clinically important outcome, gut overgrowth is defined as ≥105 potential pathogens including ‘abnormal’ aerobic Gram-negative bacilli (AGNB), ‘normal’ bacteria and yeasts, per mL of digestive tract secretion. Surveillance samples of throat and gut are the only samples to detect overgrowth. Gut overgrowth is the crucial event which precedes both primary and secondary endogenous infection, and a risk factor for the development of de novo resistance. Selective decontamination of the digestive tract (SDD) is an antimicrobial prophylaxis designed to control overgrowth.
Methods
There have been 65 randomised controlled trials of SDD in 15,000 patients over 25 years and 11 meta-analyses, which are reviewed.
Results and conclusions
These trials demonstrate that the full SDD regimen using parenteral and enteral antimicrobials reduces lower airway infection by 72 %, blood stream infection by 37 %, and mortality by 29 %. Resistance is also controlled. Parenteral cefotaxime which reaches high salivary and biliary concentrations eradicates overgrowth of ‘normal’ bacteria such as Staphylococcus aureus in the throat. Enteral polyenes control ‘normal’ Candida species. Enteral polymyxin and tobramycin, eradicate, or prevent gut overgrowth of ‘abnormal’ AGNB. Enteral vancomycin controls overgrowth of ‘abnormal’ methicillin-resistant S. aureus. SDD controls overgrowth by achieving high antimicrobial concentrations effective against ‘normal’ and ‘abnormal’ potential pathogens rather than by selectivity.
Similar content being viewed by others
Introduction
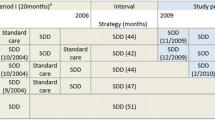
In 1996, Konrad Falke invited us to write an update on selective decontamination of the digestive tract (SDD), in particular, to explain the results of randomised controlled trials (RCTs) not showing a benefit of the technique [1]. He was convinced that negative RCTs teach more than positive ones. Over 50 RCTs had been published up to 2003 when Mervyn Singer encouraged us to write a follow-up. He thought that the reader would benefit if we compared the evidence of efficacy, safety and costs of SDD with traditional parenteral antibiotic-only approach to control infection in the intensive care unit (ICU) [2]. By the end of 2011, there have been 65 RCTs [3–67] (Fig. 1) and 11 meta-analyses of only RCTs on SDD [68–78] (Table 1) in approximately 15,000 patients over a period of 25 years. The full protocol using parenteral and enteral antimicrobials has been assessed in one-third of RCTs [5, 10, 11, 16–19, 23, 31, 32, 34, 37, 41, 47, 49, 53, 57–59, 61, 63, 66]. Among the 65 RCTs, 52 are from Europe and 13 from non-European countries (1 South America, 1 China, 2 Africa, 9 North America). The Netherlands (15), Spain (10) and Britain (8) are the leading countries from Europe. All except one meta-analyses are European (1 from The Netherlands and 9 from Italy). Massimo Antonelli convinced us that the time has come to write a third review on SDD. The emphasis would be on the mechanism of action of SDD that underlies the significant reduction of severe infections of lower airways and blood and the survival benefit with resistance being controlled.
Definitions
Carriage or carrier state exists when the same potential pathogen is isolated from at least two consecutive surveillance samples in any concentration over a period of at least 1 week. Low grade carriage is defined as <105 potential pathogens per millilitre or gram of digestive tract secretions. High grade carriage or overgrowth is defined as ≥105 potential pathogens per millilitre or gram of digestive tract secretions. Overgrowth is a risk factor for developing infection and resistance [79, 80].
SDD is an antimicrobial prophylaxis using parenteral and enteral antimicrobials. It prevents endogenous infections of lower airways and blood and reduces mortality in ICU patients [81].
SDD is based on the observation that critical illness profoundly affects the body flora, both qualitatively and quantitatively, promoting a shift from (1) normal to abnormal carriage, and (2) low to high grade carriage (overgrowth) of ‘normal’ and ‘abnormal’ flora [79–81]. There are five microorganisms that belong to the ‘normal’ flora as they are carried by healthy individuals: Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis are carried in the throat; Escherichia coli is carried in the gut; and Staphylococcus aureus and Candida albicans are carried in both throat and gut. There are nine ‘abnormal’ bacteria carried by individuals who suffer from underlying diseases: they include eight aerobic Gram-negative bacilli (AGNB) (Klebsiella, Enterobacter, Citrobacter, Proteus, Morganella, Serratia, Acinetobacter and Pseudomonas species), and methicillin-resistant Staphylococcus aureus (MRSA). ‘Abnormal’ bacteria are carried in both throat and gut [80]. In 1969, Johanson et al. [82] demonstrated that the main factor associated with oropharyngeal AGNB carriage was the severity of illness. Similarly, Chang et al. [83] showed that in cirrhotic patients the severity of liver disease was independently associated with MRSA carriage.
Detection of gut carriage and overgrowth
The traditional microbiological approach of obtaining and culturing diagnostic samples, such as tracheal aspirate and urine, can never detect overgrowth, as these samples only confirm the clinical diagnosis of infection, and its preceding stage of colonisation. Surveillance samples of throat and gut are the only samples that allow the detection of overgrowth [84]. The standard procedure includes, albeit not mandatory, a broth-enrichment stage to detect very low concentrations of micro-organisms [85, 86].
Critical illness related carriage in overgrowth concentrations (CIRCO)
CIRCO is common on ICU admission [87]. Primary endogenous infections are the most frequent ICU infections (approximately 55 %). They are caused by both normal and abnormal potential pathogens imported into the ICU by the patient’s admission flora in overgrowth concentrations. These infections generally occur early, during the first week of ICU treatment. Normal potential pathogens are the etiological agents in previously healthy individuals requiring intensive care following an acute event, such as (surgical) trauma, pancreatitis, acute hepatic failure, and burns. Abnormal bacteria can cause primary endogenous infections in patients with previous underlying disease, such as chronic obstructive pulmonary disease. Patients transferred from another ward/hospital or nursing home belong to this category.
CIRCO often develops during treatment in ICU [88]. Apart from critical illness, opiates, histamine2-receptor antagonists, and antimicrobials promote gut overgrowth by reducing peristalsis [89], increasing the gastric pH >4 [90, 91], and suppressing the normal indigenous, mainly anaerobic, flora required to control abnormal flora [92], respectively. Secondary endogenous infections are invariably caused by the nine ‘abnormal’ bacteria, accounting for one-third of ICU infections. These infections, generally, occur late, after 1 week of ICU treatment. These abnormal bacteria are first acquired in the oropharynx, and subsequently in stomach and gut. CIRCO readily develops in both oropharynx and gut.
Obviously, CIRCO is not the issue in exogenous infections, i.e. without previous carriage. Exogenous infections (approximately 15 %) are invariably caused by ‘abnormal’ bacteria, and may occur at any time during ICU treatment. Typical examples are lower airway infection caused by Acinetobacter or Pseudomonas following the use of contaminated ventilation equipment. Surveillance samples are negative for the potential pathogens that readily appear in diagnostic samples [75] (Table 2).
Gut overgrowth harms the critically ill
Gut overgrowth, in particular of AGNB, has been acknowledged to cause immuno-suppression and generalised inflammation [93]. Gut overgrowth is the crucial event preceding endogenous infections and is a risk factor for the development of de novo antimicrobial resistance. There is a qualitative and quantitative relationship between surveillance cultures of throat and rectum, and diagnostic cultures of lower airway secretions and blood [85, 88].
Dynamics of antibiotic resistance are driven by three mechanisms:
-
1.
Importation. The patient is admitted to the ICU with resistant micro-organisms in overgrowth concentrations in the gut [87];
-
2.
Acquisition from other patients with overgrowth following transmission via the hands of carers. Thirty-three percent of patients admitted as normal carriers to a medical/surgical ICU developed abnormal carriage of multi-drug-resistant K. pneumoniae and/or A. baumannii, the two abnormal AGNB endemic in the ICU during the study [94]. A higher severity of illness score on admission was a significant risk factor. Similar results were reported by Spanish researchers [95].
-
3.
De novo development. Gut overgrowth has been identified as a risk factor for the development of de novo resistance [96]. The gut of the critically ill patient with microbial overgrowth is the ideal site for the de novo development of new clones, following increased spontaneous mutation, termed hypermutation. In hypermutation, microbial populations start mutating vigorously at random, presumably as an adaptive mechanism that may cause a mutant to arise that would enable them to overcome the unfavourable surroundings, resulting in polyclonality. A high proportion of long-term ICU patients receive parenteral antimicrobials, which are invariably excreted via the bile into the gut. Although low and fluctuating, the antibiotic levels will kill sensitive clones, but allow mutating ones to become resistant to antibiotics [97]. Overgrowth not only promotes mutation but also increases the probability of transfer of genes coding for resistance between micro-organisms.
Each mechanism is responsible for approximately one-third of the resistance problem, the common denominator being overgrowth.
Control of overgrowth
In the mid-1970s, Bodey [98] realised that many systemic antimicrobials may sterilise lungs, blood and bladder but often fail to eradicate identical potential pathogens in overgrowth concentrations from the throat and/or gut.
A classical study by Bodey was the assessment of the old parenteral antifungal 5-fluorocytosine in eradicating Candida carriage [98]. He wrote that “5-fluorocytosine has substantially reduced the proportion of patients with persistent fungi in their stools and throats”. However, resistance readily occurred. Similarly, systemic prophylaxis with fluconazole also failed to eradicate yeast gut overgrowth [99], probably due to low biliary concentrations of fluconazole [100]. Bodey introduced the enteral administration of polyenes, nystatin and amphotericin B, to control fungal overgrowth [101–103]. Faecal specimens of healthy volunteers contained nystatin concentrations of <100 mg/L of faeces following the daily intake of 8 × 106 units of nystatin [102]. The faecal samples of healthy individuals taking 2,000 mg of amphotericin B daily showed 60 mg/L of faeces of amphotericin B [103]. These faecal levels are due to the high faecal binding of polyenes (Table 3).
Bodey was also the first to assess the enteral antimicrobials polymyxin E and tobramycin in controlling AGNB carriage [104]. The combination of polymyxin [105] and tobramycin [106] was chosen because it covers most abnormal AGNB, including Pseudomonas species, and is an in vitro synergistic combination [107]. Compared to polymyxin, tobramycin is less inactivated by mucosal cells, fibres and faeces [108]. Faecal specimens contained tobramycin levels of minimally 100 mg/L of faeces following the daily intake of 300 mg of tobramycin (Table 3) [109]. The faecal samples of individuals taking 600 mg of tobramycin daily showed ≥500 mg/L of faeces [110]. Polymyxin is moderately inactivated by faeces and, hence, faecal concentrations vary. Polymyxin was not detected in one-third of individuals who took 600 mg of polymyxin daily. One-third had faecal levels exceeding 1,000 mg/L of faeces, whereas the remaining individuals showed polymyxin concentrations between 16 and 1,000 mg/L of faeces [111].
At the beginning of the 1980s, Stoutenbeek, in designing the SDD protocol, searched for a parenteral antimicrobial with adequate spectrum and pharmacokinetic properties. Cefotaxime was chosen because:
-
i.
Its spectrum included both ‘normal’ and most ‘abnormal’ bacteria [112];
-
ii.
Its pharmacokinetic properties included a high excretion in saliva and bile, possibly associated with eradication of overgrowth [113]. Salivary and biliary samples were obtained from adult patients requiring biliary surgery and receiving 1 g of cefotaxime intravenously four times daily. High concentrations were measured: 6 mg/L of saliva and 20 mg/L of bile (Table 3).
Stoutenbeek subsequently evaluated the decontaminating properties of cefotaxime in trauma patients rendered free from yeasts and AGNB following the administration of enteral amphotericin B and polymyxin E/tobramycin in throat and gut [114]. Cefotaxime was found to eradicate oropharyngeal overgrowth of ‘normal’ bacteria such as S. aureus, H. influenzae, S. pneumoniae and M. catarrhalis. This original finding was confirmed by German researchers [115].
Parenteral antibiotics active against P. aeruginosa include ceftazidime, ciprofloxacin, piperacillin-tazobactam and meropenem. None of these anti-pseudomonal agents have ever been shown to clear P. aeruginosa from the throat and/or gut following intravenous administration [116, 117].
MRSA endemicity is defined as at least one new case per month of MRSA infection. Under these circumstances, the enteral SDD prophylaxis may be extended by enteral vancomycin. In early 2000, Silvestri et al. [118] observed that parenteral vancomycin failed to clear MRSA carriage from throat and gut, whilst enteral vancomycin eradicated MRSA gut carriage, and was effective in controlling a MRSA outbreak. Two grams of enteral vancomycin lead to faecal vancomycin levels of up to 24,000 mg/L of stool (Table 3) [119]. In contrast, 2 g of parenteral vancomycin were associated with stool vancomycin concentrations varying between 3 and 95 mg/L [120].
The inability to control exogenous infections is an inherent limitation of SDD. Indeed, tracheotomised patients can acquire abnormal bacteria directly into the tracheal site via the tracheostomy, without previous oropharyngeal carriage. A South African SDD RCT, in which there were 24 and 26 exogenous infections due to A. baumannii in SDD and controls, respectively, was the first to demonstrate the failure to control exogenous infections [29]. Up to 40 % of patients received a tracheostomy [121, 122]. In 2000, Morar et al. made the original observation that exogenous infections due to abnormal bacteria can be controlled by the topical application of antimicrobials. They prevented exogenous infections in tracheotomised patients by applying 0.5 g of a paste containing 2 % polymyxin E/tobramycin and 4 % vancomycin four times a day onto the tracheostomy [123, 124]. Topical antimicrobials are not part of the routine SDD protocol, but they are added to parenteral and enteral antimicrobials in case of endemicity of exogenous infections.
Clinical impact of SDD using enteral antimicrobials for control of overgrowth
The immuno-suppression reverted to normal in animals which were successfully decontaminated. SDD, after the initial experimental burn injury to rats, decreased sensitivity to a second infectious challenge of S. pneumoniae and indirectly decreased the cardiac inflammation and dysfunction associated with a septic challenge [125]. Patients who were free from AGNB following the enteral intake of polymyxin and tobramycin were able to control generalised inflammation [126]. Gut overgrowth of abnormal AGNB is the major source of endotoxin in the human body yielding up to 10 mg of endotoxin per gram of faeces [127]. The enteral antimicrobials of polymyxin/tobramycin significantly reduce the faecal endotoxin load by a factor of 104 [128]. It has been suggested that SDD may reinforce the anti-inflammatory effects of corticosteroids [129].
Enteral antimicrobials control overgrowth preventing colonisation and infection of the normally sterile internal organs. As a first step of a pneumonia prevention study, Stoutenbeek et al. [114] administered a 10-ml suspension of polymyxin E 100 mg, tobramycin 80 mg and amphotericin B 500 mg by nasogastric tube four times daily in 17 trauma patients. Ten patients (59 %) developed 13 lower airway infections, 10 primary and 3 secondary endogenous infections. P. aeruginosa and A. baumannii caused the secondary endogenous and S. pneumoniae, H. influenzae, M. catarrhalis and S. aureus were responsible for the primary endogenous lower airway infections. SDD of stomach and gut did not affect pneumonia [130].
Stoutenbeek’s second step was the assessment of the efficacy of enteral antimicrobials applied in both oropharynx and gut on the pneumonia rate [114]. Twenty-five trauma patients each received daily 2 g of a 2 % polymyxin/tobramycin/amphotericin B paste applied in the oropharynx combined with 40 ml of a solution of the same antimicrobials into the stomach and gut, divided into 4 doses. The pneumonia rate was 52 %; 13 patients developed a total of 13 lower airway infections, all of them were invariably due to ‘normal’ bacteria. Although the overall reduction was not significant, it was striking that secondary endogenous pneumonias due to ‘abnormal’ AGNB were completely prevented by the oropharyngeal decontamination, which eradicated oropharyngeal overgrowth of AGNB [131].
The third and final step of the pneumonia prophylaxis study [114] involved 63 trauma patients, who received enteral antimicrobials in throat/gut combined with parenteral cefotaxime (50–100 mg/Kg/day) to eradicate oropharyngeal overgrowth of ‘normal’ bacteria. Five patients (8 %) developed exogenous lower airway infections, primary endogenous infections disappeared, and there were no secondary endogenous lower airway infections. Six of the 11 meta-analyses had the endpoint of pneumonia (Table 1) [68, 69, 71, 74, 76, 78], and all invariably demonstrated a significant pneumonia reduction due to both Gram-positive and Gram-negative bacteria. The meta-analysis from the Italian Cochrane Centre demonstrated that enteral and parenteral antimicrobials of SDD reduced lower airway infections by 72 % [odds ratio (OR) 0.28; 95 % confidence interval (CI) 0.20–0.38] [76]. Lower airway infections due to both Gram-negative and Gram-positive bacteria were reduced by 89 % (OR 0.11; 95 % CI 0.05–0.20) and 48 % (OR 0.52; 95 % CI 0.34–0.78), respectively [74]. Interestingly, the use of the full protocol of parenteral and enteral antimicrobials was more effective in reducing Gram-negative lower airway infections than solely enteral antimicrobials (OR 0.07; 95 % CI 0.04–0.13, and OR 0.28; 95 % CI 0.11–0.68, respectively). Additionally, a recent meta-analysis showed that SDD reduces ventilator-associated tracheobronchitis by 46 % (OR 0.54; 95 % CI 0.42–0.69) [78].
In 1989, 5 years after Stoutenbeek published the first SDD study [79], Langer et al. reported their landmark RCT on pneumonia prevention [132]. No statistically different rates of pneumonia or death were found amongst three groups receiving either intravenous cefoxitin, penicillin G, or no antibiotic. Stoutenbeek explained this failure by the omission of oropharyngeal and intestinal antimicrobials [133]. In leaving overgrowth intact, resistance against cefoxitin and penicillin G readily developed followed by lethal superinfections. Liberati et al. [76] confirmed the validity of Stoutenbeek’s revolutionary concept that only the combination of parenteral and enteral antimicrobials impacts morbidity and mortality in critically ill ICU patients.
Bloodstream infection was the endpoint of three meta-analyses [72–74] (Table 1). Bloodstream infections due to AGNB were significantly reduced (OR 0.36, 95 % CI 0.22–0.60) [73], fungaemia was also reduced (OR 0.89, 95 % CI 0.16–4.95) but not significantly due to the low event rate in the control group [72]. Although Gram-positive bloodstream infections increased due to the SDD spectrum of activity primarily being against AGNB, this was not significant (OR 1.03, 95 % CI 0.75-1.41) [74].
de la Cal et al. [18] demonstrated that SDD provided a significant survival benefit in burn patients. There are only three RCTs of SDD in burn patients [3, 7, 18]; a recent meta-analysis recruiting 440 patients (289 SDD, 151 controls) showed that SDD significantly reduced mortality by 78 % (OR 0.22; 95 % CI 0.12–0.43; p < 0.001) [134].
The largest RCT to date is Dutch, and includes 6,000 patients [19]. It compares SDD and selective oropharyngeal decontamination (SOD), a modified SDD protocol without the parenteral and gut component, with standard care. The main endpoint was mortality. Both SDD and SOD significantly reduced mortality compared to standard care (OR 0.83 p = 0.02, and 0.86 p = 0.045, respectively). Although this RCT was the first to demonstrate a survival benefit of SOD, the mortality reduction was higher, albeit not significantly, with SDD than SOD. Additionally, a recent meta-analysis, including nine SOD RCTs and 4,733 patients, failed to show any significant reduction in mortality (OR 0.93; 95 % CI 0.81–1.07) [135]. In contrast, an Italian meta-analysis, including only RCTs using the full SDD protocol, showed a mortality reduction of 29 % (OR 0.71; 95 % CI 0.61–0.82) [75]. This effect achieved a 42 % mortality reduction in studies where carriage was eradicated (OR 0.58; 95 % CI 0.45–0.77).
Remarkably, the design of the study determines the magnitude of the survival benefit of SDD. In the study in which all eligible patients received the full SDD protocol, the significant reduction in the OR for mortality was 40 % [17]. If half the patients received SDD due to the RCT design, the significant reduction in the OR for mortality was 29 % [75]. In the most recent Dutch study, one-third of the patients received SDD and reduction in the OR for mortality was still significant, albeit at 17 % [19]. As a practical guideline, SDD should be applied to all ventilated patients of the unit, as mixing non-decontaminated with decontaminated patients still allows transmission of potential pathogens and hence exogenous infections in successfully decontaminated patients.
Impact of SDD on resistance
The main category of potential pathogens in which antimicrobial resistance is a problem in the ICU are AGNB. There are two scenarios:
-
1.
AGNB sensitive to the decontaminating agents polymyxin/tobramycin.
de Jonge et al. [17] conducted an RCT in 934 critically ill adult patients. The in-hospital mortality rate was significantly lower for SDD compared with controls (24 vs. 31 %; p = 0.002). Carriage of AGNB resistant to polymyxin E, tobramycin, ceftazidime, ciprofloxacin and imipenem was significantly reduced in SDD patients compared with controls (16 vs. 26 %; p = 0.001). Similarly, de Smet et al. [19] showed that there were fewer patients with AGNB in rectal swabs resistant to the marker antibiotics in the SDD than the SOD group. Additionally, bloodstream infections due to highly resistant pathogens was significantly reduced by SDD compared with SOD (OR 0.37, 95 % CI 0.16–0.85) [136].
-
2.
AGNB resistant to decontaminating agents polymyxin/tobramycin.
-
Serratia spp are the only potential pathogens intrinsically resistant to polymyxin/tobramycin. In the case of endemic Serratia, polymyxin/tobramycin should be replaced by polymyxin/paromomycin or gentamicin [104].
-
Extended-spectrum beta-lactamase (ESBL) producing AGNB are often resistant to tobramycin but always sensitive to polymyxin [137]. In the case of endemicity of ESBL-producing AGNB, tobramycin may be replaced by an active aminoglycoside [138].
-
The concept of exposing vast numbers of critically ill patients to broad-spectrum antibiotics runs counter to existing theoretical models (and dogma) related to the genesis and promotion of antimicrobial resistance in pathogens acquired in the healthcare setting [139]. Indeed, the experts are concerned that SDD may lead to an ecological catastrophe. In contrast, the best evidence is that long-term use of SDD is safe. It actually reduces resistance rather than increasing it [140]. Indeed, traditionalists reject this evidence for four reasons: (1) the absence of resistance is counterintuitive; (2) the evidence comes from ICUs with an unusually low level of resistance; (3) the observation period is too short; and (4) many resistant potential pathogens are not covered by the SDD prophylaxis [141]. We would like to counteract these four arguments [142]. The first argument results from experience of only parenteral, rather than enteral, antibiotic use in the ICU. Critically ill patients invariably have overgrowth of potential pathogens with a high capacity for antimicrobial resistance, exacerbated by the excretion of most parenteral antimicrobials via bile in sublethal concentrations. In contrast, the aim of enteral antimicrobials of the SDD protocol, particularly polymyxin/tobramycin, is the eradication of overgrowth, the major risk factor for the emergence of antimicrobial resistance in particular against cefotaxime. Several European ICUs have implemented SDD for over 20 years. None have reported an outbreak of infection due to micro-organisms resistant to SDD. Finally, SDD has been assessed in ICUs with endemic vancomycin-resistant enterococci and Aspergillus fumigatus. Investigators reported no difference between test and control groups [6, 30]. American experts [143] consider the Oostdijk study [144] as proof that SDD causes resistance. However, that study is a point-prevalence survey in which all patients in the unit, whether enrolled or not in the study, were included. This type of ecological study is labelled as a low level evidence study (2C) [145, 146].
SDD use is increasing in Europe, in contrast, its use is uncommon in the United States. We believe the main reason is the “primacy of opinion over evidence” [147, 148]. Indeed, there are opinion leaders who assert that SDD does not provide a survival benefit, whilst it promotes an ecological resistance disaster [148]. The other major impediment to the widespread use of SDD is the lack of support by any pharmaceutical company, explaining why paste, gel and suspension of SDD are not available on the shelf [148].
Selectivity is not required
There is still some uncertainty of how SDD achieves its significant benefits [149]. It now seems clear that ‘SDD’ was an inappropriate choice of terminology. Rather than selectively removing aerobic bacteria and leaving the anaerobic intestinal microbes unaffected, as the name misleadingly implies, SDD actually works by achieving high antimicrobial concentrations effective against overgrowth of both normal and abnormal flora. It is a contradiction in terms to be both selective and yet achieve effective decontamination or eradication of aerobic overgrowth. However, the term SDD is now so well established that to change it would cause too much confusion. It has been hypothesised that eradication of aerobic overgrowth would lower the rate of oxygen consumption permitting an increase in pO2 of the gut lumen content from 5 to 60 mmHg: under such conditions, strictly anaerobic micro-organisms can no longer survive even though they may not themselves be sensitive to the decontaminating agents [150].
Future lines of SDD research
Future lines of SDD research may include (1) the impact of the traditional SDD protocol of polymyxin/tobramycin on ICUs to which patients who may carry multi-resistant AGNB are regularly admitted [151]; (2) the impact of polymyxin/tobramycin/vancomycin on ICUs with endemic vancomycin resistant enterococci [6, 30]; (3) the impact of the traditional SDD protocol of polymyxin/tobramycin/amphotericin B on ICUs to which neutropenic patients with mucositis are regularly admitted [152]; and (4) the impact of overgrowth control on the severity of the systemic inflammatory response syndrome [77, 126, 153].
Conclusion
SDD is the most studied manoeuvre in intensive care medicine. There have been 65 RCTs of SDD, in about 15,000 patients, and 11 meta-analyses of RCTs, over a period of 25 years. SDD using parenteral and enteral antimicrobials has been shown to reduce lower airway infection by 72 %, bloodstream infection by 37 %, and mortality by 29 %, with resistance being controlled.
SDD controls overgrowth by achieving high antimicrobial concentrations effective against ‘normal’ and’ abnormal’ potential pathogens rather than by selectivity. However, whatever its precise mechanism, withholding SDD from critically ill patients must now surely be ethically questionable given the vast evidence base.
References
Baxby D, van Saene HKF, Stoutenbeek CP, Zandstra DF (1996) Selective decontamination of the digestive tract: 13 years on, what it is and what it is not. Intensive Care Med 22:699–706
van Saene HKF, Petros AJ, Ramsay G, Baxby D (2003) All great truths are iconoclastic: selective decontamination of the digestive tract moves from heresy to level 1 truth. Intensive Care Med 29:677–690
Abdel-Razek SM, Abdel-Khalek AH, Allam AM, Shalaby H, Mandoor S, Higazi M (2000) Impact of selective gastrointestinal decontamination on mortality and morbidity in severely burned patients. Ann Burns Fire Disast 13:213–216
Abele-Horn M, Dauber A, Bauernfeind A, Russwurm W, Seyfarth-Metzger I, Gleich P, Ruckdeschel G (1997) Decrease in nosocomial pneumonia in ventilated patients by selective oropharyngeal decontamination (SOD). Intensive Care Med 23:187–195
Aerdts SJ, van Dalen R, Clasener HA, Festen J, van Lier HJ, Vollaard EJ (1991) Antibiotic prophylaxis of respiratory tract infection in mechanically ventilated patients: a prospective, blinded, randomized trial of the effect of a novel regimen. Chest 100:783–791
Arnow PM, Carandang GC, Zabner R, Irwin ME (1996) Randomized controlled trial of selective bowel decontamination for prevention of infections following liver transplantation. Clin Infect Dis 22:997–1003
Barret JP, Jeschke MG, Herndon DN (2001) Selective decontamination of the digestive tract in severely burned pediatric patients. Burns 27:439–445
Bergmans DC, Bonten MJ, Gaillard CA, Paling JC, van der Geest S, van Tiel FH, Beysens AJ, de Leeuw PW, Stobberingh EE (2001) Prevention of ventilator-associated pneumonia by oral decontamination: a prospective, randomized, double-blind, placebo-controlled study. Am J Respir Crit Care Med 164:382–388
Bion JF, Badger I, Crosby HA, Hutchings P, Kong KL, Baker J, Hutton P, McMaster P, Buckels JA, Elliott TS (1994) Selective decontamination of the digestive tract reduces gram-negative pulmonary colonization but not systemic endotoxemia in patients undergoing elective liver transplantation. Crit Care Med 22:40–49
Blair P, Rowlands BJ, Lowry K, Webb H, Armstrong P, Smilie J (1991) Selective decontamination of the digestive tract: a stratified, randomized, prospective study in a mixed intensive care unit. Surgery 110:303–310
Boland JP, Sadler DL, Stewart WA, Wood DJ, Zerick W, Snodgrass KR (1991) Reduction of nosocomial respiratory tract infections in multiple trauma patient requiring mechanical ventilation by selective parenteral and enteral antisepsis regimen [SPEAR] in the intensive care unit. In: 17th International Congress of Chemotherapy, Berlin, Abstract 0465
Bouter H, Schippers EF, Luelmo SA, Versteegh MI, Ros P, Guiot HF, Frolich M, van Dissel JT (2002) No effect of preoperative selective gut decontamination on endotoxemia and cytokine activation during cardiopulmonary bypass: a randomized, placebo-controlled study. Crit Care Med 30:38–43
Brun-Buisson C, Legrand P, Rauss A, Richard C, Montravers F, Besbes M, Meakins JL, Soussy CJ, Lemaire F (1989) Intestinal decontamination for control of nosocomial multiresistant gram-negative bacilli: study of an outbreak in an intensive care unit. Ann Intern Med 110:873–881
Camus C, Bellissant E, Sebille V, Perrotin D, Garo B, Legras A, Renault A, Le Corre P, Donnio PY, Gacouin A, Le Tulzo Y, Thomas R (2005) Prevention of acquired infections in intubated patients with the combination of two decontamination regimens. Crit Care Med 33:307–314
Cerra FB, Maddaus MA, Dunn DL, Wells CL, Konstantinides NN, Lehmann SL, Mann HJ (1992) Selective gut decontamination reduces nosocomial infections and length of stay but not mortality or organ failure in surgical intensive care unit patients. Arch Surg 127:163–169
Cockerill FR 3rd, Muller SR, Anhalt JP, Marsh HM, Farnell MB, Mucha P, Gillespie DJ, Ilstrup DM, Larson-Keller JJ, Thompson RL (1992) Prevention of infection in critically ill patients by selective decontamination of the digestive tract. Ann Intern Med 117:545–553
de Jonge E, Schultz MJ, Spanjaard L, Bossuyt PM, Vroom MB, Dankert J, Kesecioglu J (2003) Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet 362:1011–1016
de la Cal MA, Cerdá E, García-Hierro P, van Saene HK, Gómez-Santos D, Negro E, Lorente JA (2005) Survival benefit in critically ill burned patients receiving selective decontamination of the digestive tract: a randomized, placebo-controlled, double-blind trial. Ann Surg 241:424–430
de Smet AM, Kluytmans JA, Cooper BS, Mascini EM, Benus RF, van der Werf TS, van der Hoeven JG, Pickkers P, Bogaers-Hofman D, van der Meer NJ, Bernards AT, Kuijper EJ, Joore JC, Leverstein-van Hall MA, Bindels AJ, Jansz AR, Wesselink RM, de Jongh BM, Dennesen PJ, van Asselt GJ, te Velde LF, Frenay IH, Kaasjager K, Bosch FH, van Iterson M, Thijsen SF, Kluge GH, Pauw W, de Vries JW, Kaan JA, Arends JP, Aarts LP, Sturm PD, Harinck HI, Voss A, Uijtendaal EV, Blok HE, Thieme Groen ES, Pouw ME, Kalkman CJ, Bonten MJ (2009) Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med 360:20–31
Diepenhorst GM, van Ruter O, Besselink MG, van Santvoort HC, Wijnandts PR, Renooij W, Gouma DJ, Gooszen HG, Boermeester MA (2011) Influence of prophylactic probiotics and selective decontamination on bacterial translocation in patients undergoing pancreatic surgery: a randomized controlled trial. Shock 35:9–16
Farran L, Llop J, Sans M, Kreisler E, Miró M, Galan M, Rafecas A (2008) Efficacy of enteral decontamination in the prevention of anastomotic dehiscence and pulmonary infection in esophagogastric surgery. Dis Esophagus 21:159–164
Ferrer M, Torres A, González J, Puig de la Bellacasa J, El-Ebiary M, Roca M, Gatell JM, Rodriguez-Roisin R (1994) Utility of selective digestive decontamination in mechanically ventilated patients. Ann Intern Med 120:389–395
Finch RG, Tomlinson P, Holliday M, Sole K, Stack C, Rocker G (1991) Selective decontamination of the digestive tract (SDD) in the prevention of secondary sepsis in a medical/surgical intensive care unit. In: 17th International Congress of Chemotherapy, Berlin, Abstract 0474
Flaherty J, Nathan C, Kabins SA, Weinstein RA (1990) Pilot trial of selective decontamination for prevention of bacterial infection in an intensive care unit. J Infect Dis 162:1393–1397
Gastinne H, Wolff M, Delatour F, Faurisson F, Chevret S (1992) A controlled trial in intensive care units of selective decontamination of the digestive tract with nonabsorbable antibiotics. The French Study Group on Selective Decontamination of the Digestive Tract. N Engl J Med 326:594–599
Gaussorgues PH, Salord F, Sirodot M, Tigaud S, Cagin S, Gerard M, Robert D (1991) Efficacite de la decontamination digestive sur la survenue des bacteriemies nosocomiales chez les patients sous ventilation mecanique et recevant des betamimetiques. Rean Soins Intens Med Urg 7:169–174
Georges B, Mazerolles M, Decun JF, Rouge P, Pomies S, Cougot P, Andrieu P, Virenque CH (1994) Décontamination digestive sélective. Résultats d’une étude chez le polytraumatisé. Rean Urg 3:621–627
Gosney M, Martin MV, Wright AE (2006) The role of selective decontamination of the digestive tract in acute stroke. Age Ageing 35:42–47
Hammond JM, Potgieter PD, Saunders GL, Forder AA (1992) Double-blind study of selective decontamination of the digestive tract in intensive care. Lancet 340:5–9
Hellinger WC, Yao JD, Alvarez S, Blair JE, Cawley JJ, Paya CV, O’Brien PC, Spivey JR, Dickson RC, Harnois DM, Douglas DD, Hughes CB, Nguyen JH, Mulligan DC, Steers JL (2002) A randomized, prospective, double-blinded evaluation of selective bowel decontamination in liver transplantation. Transplantation 73:1904–1909
Jacobs S, Foweraker JE, Roberts SE (1992) Effectiveness of selective decontamination of the digestive tract (SDD) in an ICU with a policy encouraging a low gastric pH. Clin Intensive Care 3:52–58
Kerver AJ, Rommes JH, Mevissen-Verhage EA, Hulstaert PF, Vos A, Verhoef J, Wittebol P (1988) Prevention of colonization and infection in critically ill patients: a prospective randomized study. Crit Care Med 16:1087–1093
Korinek AM, Laisne MJ, Nicolas MH, Raskine L, Deroin V, Sanson-Lepors MJ (1993) Selective decontamination of the digestive tract in neurosurgical intensive care unit patients: a double-blind, randomized, placebo-controlled study. Crit Care Med 21:1466–1473
Krueger WA, Lenhart FP, Neeser G, Ruckdeschel G, Schreckhase H, Eissner HJ, Forst H, Eckart J, Peter K, Unertl KE (2002) Influence of combined intravenous and topical antibiotic prophylaxis on the incidence of infections, organ dysfunctions, and mortality in critically ill surgical patients: a prospective, stratified, randomized, double-blind, placebo-controlled clinical trial. Am J Respir Crit Care Med 166:1029–1037
Laggner AN, Tryba M, Georgopoulos A, Lenz K, Grimm G, Graninger W, Schneeweiss B, Druml W (1994) Oropharyngeal decontamination with gentamicin for long-term ventilated patients on stress ulcer prophylaxis with sucralfate? Wien Klin Wochenschr 106:15–19
Lingnau W, Berger J, Javorsky F, Lejeune P, Mutz N, Benzer H (1997) Selective intestinal decontamination in multiple trauma patients: prospective, controlled trial. J Trauma 42:687–694
Luiten EJ, Hop WC, Lange JF, Bruining HA (1995) Controlled clinical trial of selective decontamination for the treatment of severe acute pancreatitis. Ann Surg 222:57–65
Martinez-Pellús AE, Merino P, Bru M, Conejero R, Seller G, Muñoz C, Fuentes T, Gonzalez G, Alvarez B (1993) Can selective digestive decontamination avoid the endotoxemia and cytokine activation promoted by cardiopulmonary bypass? Crit Care Med 21:1684–1691
Martinez-Pellús AE, Merino P, Bru M, Canovas J, Seller G, Sapiña J, Fuentes T, Moro J (1997) Endogenous endotoxemia of intestinal origin during cardiopulmonary bypass. Role of type of flow and protective effect of selective digestive decontamination. Intensive Care Med 23:1251–1257
Oudhuis GJ, Bergmans DC, Dormans T, Zwaveling JH, Kessels A, Prins MH, Stobberingh EE, Verbon A (2011) Probiotics versus antibiotic decontamination of the digestive tract: infection and mortality. Intensive Care Med 37:110–117
Palomar M, Alvarez-Lerma F, Jorda R, Bermejo B, Catalan study group of nosocomial pneumonia prevention (1997) Prevention of nosocomial infection in mechanically ventilated patients: selective digestive decontamination versus sucralfate. Clin Intensive Care 8:228–235
Pneumatikos I, Koulouras V, Nathanail C, Goe D, Nakos G (2002) Selective decontamination of subglottic area in mechanically ventilated patients with multiple trauma. Intensive Care Med 28:432–437
Pugin J, Auckenthaler R, Lew DP, Suter PM (1991) Oropharyngeal decontamination decreases incidence of ventilator-associated pneumonia: a randomized, placebo-controlled, double-blind clinical trial. JAMA 265:2704–2710
Quinio B, Albanèse J, Bues-Charbit M, Viviand X, Martin C (1996) Selective decontamination of the digestive tract in multiple trauma patients: a prospective double-blind, randomized, placebo-controlled study. Chest 109:765–772
Rayes N, Seehofer D, Hansen S, Boucsein K, Müller AR, Serke S, Bengmark S, Neuhaus P (2002) Early enteral supply of Lactobacillus and fiber versus selective bowel decontamination: a controlled trial in liver transplant recipients. Transplantation 74:123–128
Rios F, Maskin B, Saenz Valiente A, Galante A, Cazes Camarero P, Aguilar L, et al (2005) Prevention of ventilator associated pneumonia (VAP) by oral decontamination (OD). Prospective, Randomized, Double-blind, Placebo-controlled study. Am Thoracic Soc International Conf, San Diego, USA, C95; poster 608
Rocha LA, Martín MJ, Pita S, Paz J, Seco C, Margusino L, Villanueva R, Duran MT (1992) Prevention of nosocomial infection in critically ill patients by selective decontamination of the digestive tract: a randomized, double blind, placebo-controlled study. Intensive Care Med 18:398–404
Rodríguez-Roldán JM, Altuna-Cuesta A, López A, Carrillo A, Garcia J, León J, Martinez-Pellus AJ (1990) Prevention of nosocomial lung infection in ventilated patients: use of an antimicrobial pharyngeal nonabsorbable paste. Crit Care Med 18:1239–1242
Rolando N, Gimson A, Wade J, Philpott-Howard J, Casewell M, Williams R (1993) Prospective controlled trial of selective parenteral and enteral antimicrobial regimen in fulminant liver failure. Hepatology 17:196–201
Rolando N, Wade JJ, Stangou A, Gimson AE, Wendon J, Philpott-Howard J, Casewell MW, Williams R (1996) Prospective study comparing the efficacy of prophylactic parenteral antimicrobials, with or without enteral decontamination, in patients with acute liver failure. Liver Transpl Surg 2:8–13
Roos D, Dijksman LM, Oudemans-van Straaten HM, de Wit LT, Gouma DJ, Gerhards MF (2011) Randomized clinical rial of perioperative selective decontamination of the digestive tract versus placebo in elective gastrointestinal surgery. Br J Surg 98:1365–1372
Ruza F, Alvarado F, Herruzo R, Delgado MA, García S, Dorao P, Goded F (1998) Prevention of nosocomial infection in a pediatric intensive care unit (PICU) through the use of selective digestive decontamination. Eur J Epidemiol 14:719–727
Sánchez García M, Cambronero Galache JA, López Diaz J, Cerdá Cerdá E, Rubio Blasco J, Gómez Aguinaga MA, Núnez Reiz A, Rogero Marín S, Onoro Canaveral JJ, Sacristán del Castillo JA (1998) Effectiveness and cost of selective decontamination of the digestive tract in critically ill intubated patients. A randomized, double-blind, placebo-controlled, multicenter trial. Am J Respir Crit Care Med 158:908–916
Schardey HM, Joosten U, Finke U, Staubach KH, Schauer R, Heiss A, Kooistra A, Rau HG, Nibler R, Lüdeling S, Unertl K, Ruckdeschel G, Exner H, Schildberg FW (1997) The prevention of anastomotic leakage after total gastrectomy with local decontamination: a prospective, randomized, double-blind, placebo-controlled multicenter trial. Ann Surg 225:172–180
Smith SD, Jackson RJ, Hannakan CJ, Wadowsky RM, Tzakis AG, Rowe MI (1993) Selective decontamination in pediatric liver transplants: a randomized prospective study. Transplantation 55:1306–1309
Stoutenbeek CP,van Saene HKF, Zandstra DF (1996) Prevention of multiple organ system failure by selective decontamination of the digestive tract in multiple trauma patients. In: Faist E, Baue AE, Schildberg FW (eds) The immune consequences of trauma, shock and sepsis—mechanisms and therapeutic approaches. Pabst, Lengerich, pp 1055–1066
Stoutenbeek CP, van Saene HK, Little RA, Whitehead A; Working Group on Selective Decontamination of the Digestive Tract (2007) The effect of selective decontamination of the digestive tract on mortality in multiple trauma patients: a multicenter randomized controlled trial. Intensive Care Med 33:261–270
Tetteroo GW, Wagenvoort JH, Castelein A, Tilanus HW, Ince C, Bruining HA (1990) Selective decontamination to reduce gram-negative colonisation and infections after oesophageal resection. Lancet 335:704–707
Ulrich C, Harinck-de Weerd JE, Bakker NC, Jacz K, Doornbos L, de Ridder VA (1989) Selective decontamination of the digestive tract with norfloxacin in the prevention of ICU-acquired infections: a prospective randomized study. Intensive Care Med 15:424–431
Unertl K, Ruckdeschel G, Selbmann HK, Jensen U, Forst H, Lenhart FP, Peter K (1987) Prevention of colonization and respiratory infections in long-term ventilated patients by local antimicrobial prophylaxis. Intensive Care Med 13:106–113
Verwaest C, Verhaegen J, Ferdinande P, Schetz M, Van den Berghe G, Verbist L, Lauwers P (1997) Randomized, controlled trial of selective digestive decontamination in 600 mechanically ventilated patients in a multidisciplinary intensive care unit. Crit Care Med 25:63–71
Wiener J, Itokazu G, Nathan C, Kabins SA, Weinstein RA (1995) A randomized, double-blind, placebo-controlled trial of selective digestive decontamination in a medical-surgical intensive care unit. Clin Infect Dis 20:861–867
Winter R, Humphreys H, Pick A, MacGowan AP, Willatts SM, Speller DC (1992) A controlled trial of selective decontamination of the digestive tract in intensive care and its effect on nosocomial infection. J Antimicrob Chemother 30:73–87
Yilmazlar A, Ozyurt G, Kahveci F, Goral G (2009) Selective digestive decontamination can be an infection-prevention regimen for the intoxicated patients. J Pharmacol Toxicol 4:36–40
Yu J, Xiao YB, Wang XY (2007) Effects of preoperatively selected gut decontamination on cardiopulmonary bypass-induced endotoxemia. Chin J Traumatol 10:131–137
Zobel G, Kuttnig M, Grubbauer HM, Semmelrock HJ, Thiel W (1991) Reduction of colonization and infection rate during pediatric intensive care by selective decontamination of the digestive tract. Crit Care Med 19:1242–1246
Zwaveling JH, Maring JK, Klompmaker IJ, Haagsma EB, Bottema JT, Laseur M, Winter HL, van Enckevort PJ, TenVergert EM, Metselaar HJ, Bruining HA, Slooff MJ (2002) Selective decontamination of the digestive tract to prevent postoperative infection: a randomized placebo-controlled trial in liver transplant patients. Crit Care Med 30:1204–1209
Vandenbroucke-Grauls CMJ, Vandenbroucke JP (1991) Effect of selective decontamination of the digestive tract on respiratory tract infections and mortality in the intensive care unit. Lancet 338:859–862
D’Amico R, Pifferi S, Leonetti C, Torri V, Tinazzi A, Liberati A on behalf of the study investigators (1998) Effectiveness of antibiotic prophylaxis in critically ill adult patients: systematic review of randomised controlled trials. BMJ 316:1275–1285
Safdar N, Said A, Lucey MR (2004) The role of selective decontamination for reducing infection in patients undergoing liver transplantation: a systematic review and meta-analysis. Liver Transpl 10:817–827
Liberati A, D’Amico R, Pifferi S, Torri V, Brazzi L (2004) Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care (Cochrane Review). In: The Cochrane Library Issue 1. Wiley, Chichester, UK
Silvestri L, van Saene HKF, Milanese M, Gregori D (2005) Impact of selective decontamination of the digestive tract on fungal carriage and infection: systematic review of randomized controlled trials. Intensive Care Med 31:898–910
Silvestri L, van Saene HKF, Milanese M, Duri D, Gregori D, Gullo A (2007) Selective decontamination of the digestive tract reduces bloodstream infections and mortality in critically ill patients: a systematic review of randomized controlled trials. J Hosp Infect 65:187–203
Silvestri L, van Saene HKF, Casarin AL, Berlot G, Gullo A (2008) Impact of selective decontamination of the digestive tract on carriage and infection due to Gram-negative and Gram-positive bacteria: systematic review of randomized controlled trials. Anaesth Intens Care 36:324–338
Silvestri L, van Saene HKF, Weir I, Gullo A (2009) Survival benefit of the full selective digestive decontamination regimen. J Crit Care 24:474e7–474e14
Liberati A, D’Amico R, Pifferi S, Torri V, Brazzi L, Parmelli E (2009) Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database Syst Rev CD000022
Silvestri L, van Saene HKF, Zandstra DF, Marshall JC, Gregori D, Gullo A (2010) Impact of selective decontamination of the digestive tract on multiple organ dysfunction syndrome: systematic review of randomized controlled trials. Crit Care Med 38:1370–1376
Silvestri L, van Saene HKF, Zandstra DF (2010) Selective digestive decontamination reduces ventilator-associated tracheobronchitis. Respir Med 104:1953–1955
Stoutenbeek CP, van Saene HKF, Miranda DR, Zandstra DF (1984) The effect of selective decontamination of the digestive tract on colonisation and infection rate in multiple trauma patients. Intensive Care Med 10:185–192
van Saene HKF, Damjanovic V, Murray AE, de la Cal MA (1996) How to classify infections in intensive care units–the carrier state, a criterion whose time has come? J Hosp Infect 33:1–12
Silvestri L, de la Cal MA, van Saene HKF (2009) Selective decontamination of the digestive tract—Twenty-five years experience. In: Gullo A, Besso J, Lumb PD, Williams GF (eds) Intensive and critical care medicine. Springer, Italia, pp 273–283
Johanson WG, Pierce AK, Sanford JP (1969) Changing pharyngeal bacterial flora of hospitalized patients. Emergence of Gram-negative bacilli. N Engl J Med 281:1137–1140
Chang FY, Singh N, Gayowski T, Wagener MM, Marino IR (1998) Staphylococcus aureus nasal colonization in patients with cirrhosis: prospective assessment of association with infection. Infect Control Hosp Epidemiol 19:328–332
Schimpff SC (1981) Surveillance cultures. J Infect Dis 144:81–84
Van Uffelen R, van Saene HK, Fidler V, Löwenberg A (1984) Oropharyngeal flora as a source of bacteria colonizing the lower airways in patients on artificial ventilation. Intensive Care Med 10:233–237
Silvestri L, Monti Bragadin C, Milanese M, Gregori D, Consales C, Gullo A, van Saene HKF (1999) Are most ICU infections really nosocomial? A prospective observational cohort study in mechanically ventilated patients. J Hosp Infect 42:125–133
Viviani M, Van Saene HK, Pisa F, Lucangelo U, Silvestri L, Momesso E, Berlot G (2010) The role of admission surveillance cultures in patients requiring prolonged mechanical ventilation in the intensive care unit. Anaesth Intens Care 38:325–335
de la Cal MA, Cerdá E, van Saene HKF, García-Hierro P, Negro E, Parra ML, Arias S, Ballesteros D (2004) Effectiveness and safety of enteral vancomycin to control endemicity of methicillin-resistant Staphylococcus aureus in a medical/surgical intensive care unit. J Hosp Infect 56:175–183
Husebye H (1995) Gastrointestinal motility disorders and bacterial overgrowth. J Intern Med 237:419–427
Reusser P, Zimmerli W, Scheidegger D, Marbet GA, Buser M, Gyr K (1989) Role of gastric colonization in nosocomial infections and endotoxemia: a prospective study in neurosurgical patients on mechanical ventilation. J Infect Dis 160:414–421
Reusser P, Gyr K, Scheidegger D, Buchmann B, Buser M, Zimmerli W (1990) Prospective endoscopic study of stress erosions and ulcers in critically ill neurosurgical patients: current incidence and effect of acid-reducing prophylaxis. Crit Care Med 18:270–274
van Saene HKF, Stoutenbeek CP, Geitz JN, van Saene JJM, Hart CA (1988) Effect of amoxicillin on colonization resistance in human volunteers. Microb Ecol Health Dis 1:169–177
Petros AJ, Taylor N, van Saene HKF, Silvestri L (2011) Gut overgrowth harms the critically ill. Intensive Care Med 37:1560–1562
Garrouste-Orgeas M, Marie O, Rouveau M, Villiers S, Arlet G, Schlemmer B (1996) Secondary carriage with multi-resistant Acinetobacter baumannii and Klebsiella pneumoniae in an adult ICU population: relationship with nosocomial infections and mortality. J Hosp Infect 34:279–289
Pena C, Guzman A, Suarez C, Dominguez MA, Tubau F, Pujol M, Gudiol F, Ariza J (2007) Effects of carbapenem exposure on the risk for digestive tract carriage of intensive care unit-endemic carbapenem-resistant Pseudomonas aeruginosa strains in critically ill patients. Antimicrob Agents Chemother 51:1967–1971
van Saene HKF, Taylor N, Damjanovic V, Sarginson RE (2008) Microbial gut overgrowth guarantees increased spontaneous mutation leading to polyclonality and antibiotic resistance in the critically ill. Curr Drug Targets 9:419–421
Kohanski MA, DePristo MA, Collins JJ (2010) Sublethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis. Mol Cell 37:311–320
Bodey GP, Rodriguez V (1976) Protected environment–prophylactic antibiotic programmes; microbiological studies. Clin Haematol 5:395–408
Garbino J, Lew DP, Romand JA, Hugonnet S, Auckenthaler R, Pittet D (2002) Prevention of severe Candida infections in non-neutropenic, high-risk, critically ill patients: a randomized, double-blind, placebo-controlled trial in patients treated by selective digestive decontamination. Intensive Care Med 28:1708–1717
Bozzette SA, Gordon RL, Yen A, Rinaldi M, Ito MK, Fierer J (1992) Biliary concentrations of fluconazole in a patient with candidal cholecystitis: case report. Clin Infect Dis 15:701–703
Bodey GP (1969) The effect of amphotericin B on the fungal flora in feces. Clin Pharmacol Therapeutics 10:675–680
Hofstra W, de Vries-Hospers HG, van der Waaij D (1979) Concentrations of nystatin in faeces after oral administration of various doses of nystatin. Infection 7:166–170
Hofstra W, de Vries-Hospers HG, van der Waaij D (1982) Concentrations of amphotericin B in faeces and blood of healthy volunteers after the oral administration of various doses. Infection 10:223–227
Bodey GP (1981) Antibiotic prophylaxis in cancer patients: regimens of oral, nonabsorbable antibiotics for prevention of infection during induction of remission. Rev Infect Dis 3(suppl):S259–S268
Hoeprich PD (1970) The polymyxins. Med Clin North Am 54:1257–1265
Neu HC (1976) Tobramycin: an overview. J Infect Dis 134:S3–S19
Kuipers JS (1975) Combinations of antimicrobial agents. I. The in vitro sensitivity of 100 strains of Pseudomonas aeruginosa to polymyxin B, colistin, carbenicillin, gentamicin and doxycycline and to various combinations of these antibiotics. Arch Chir Neerl 27:257–270
van Saene JJ, van Saene HK, Tarko-Smit NJ, Beukeveld GJ (1988) Enterobacteriaceae suppression by three different oral doses of polymyxin E in human volunteers. Epidemiol Infect 100:407–417
Mulder JG, Wiersma WE, Welling GW, van der Waay D (1984) Low dose oral tobramycin treatment for selective decontamination of the digestive tract: a study in human volunteers. J Antimicrob Chemother 13:495–504
Bodey GP, Pan T (1980) Absorption of tobramycin after chronic oral administration. Curr Ther Res 28:394–401
Gotoff SP, Lepper MH, Fiedler MA (1965) Treatment of Salmonella carriers with colistin sulfate. Am J Med Sci 249:399–403
Neu HC, Aswapokee N, Aswapokee P, Fu KP (1979) HR 756, a new cephalosporin active against Gram-positive and Gram-negative aerobic and anaerobic bacteria. Antimicrob Ag Chemother 15:279–281
Novick WJ Jr (1982) Levels of cefotaxime in body fluids and tissues: a review. Rev Infect Dis 4(Suppl):S346–S353
Stoutenbeek CP, van Saene HKF, Miranda DR, Zandstra DF, Langrehr D (1987) The effect of oropharyngeal decontamination using topical non-absorbable antibiotics on the incidence of nosocomial respiratory tract infections in multiple trauma patients. J Trauma 27:357–364
Maier H, Zerfowski M, Schliegel P (1991) Excretion of beta-lactam antibiotics in human parotid saliva. HNO 39:102–107
Toltzis P, Yamashita T, Vilt L, Green M, Morrissey A, Spinner-Block S, Blumer J (1998) Antibiotic restriction does not alter endemic colonization with resistant gram-negative rods in a pediatric intensive care unit. Crit Care Med 26:1893–1899
Neuhauser MM, Weinstein RA, Rydman R, Danziger LH, Karam G, Quinn JP (2003) Antibiotic resistance among gram-negative bacilli in US intensive care units. JAMA 289:885–888
Silvestri L, Milanese M, Oblach L, Fontana F, Gregori D, Guerra R, van Saene HK (2002) Enteral vancomycin to control methicillin-resistant Staphylococcus aureus outbreak in mechanically ventilated patients. Am J Infect Control 30:391–399
Geraci JE, Heilman FR, Nichols DR, Wellman WE, Ross CT (1956) Some laboratory and clinical experience with a new antibiotic, vancomycin. Proc Staff Meet Mayo Clin 31:564–582
Currie BP, Lemos-Filho L (2004) Evidence for biliary excretion of vancomycin into stool during intravenous therapy: potential implications for rectal colonization with vancomycin-resistant enterococci. Antimicrob Agents Chemother 40:4427–4429
Hammond JM, Potgieter PD (1995) Is there a role for selective decontamination of the digestive tract in primarily infected patients in the ICU? Anaesth Intensive Care 23:168–174
Palmer LB, Donelan SV, Fox G, Bellemore E, Greene WH (1995) Gastric flora in chronically mechanically ventilated patients. Am J Respir Crit Care Med 151:1063–1067
Morar P, Singh V, Makura Z, Jones A, Baines P, Selby A, Sarginson R, Hughes J, van Saene R (2002) Differing pathways of lower airway colonization and infection according to mode of ventilation (endotracheal vs tracheotomy). Arch Otolaryngol Head Neck Surg 128:1061–1066
Morar P, Makura Z, Jones A, Baines P, Selby A, Hughes J, van Saene R (2000) Topical antibiotics on tracheostoma prevents exogenous colonization and infection of lower airways in children. Chest 117:513–518
Horton JW, Maass DL, White J, Minei JP (2007) Reducing susceptibility to bacteremia after experimental burn injury: a role for selective decontamination of the digestive tract. J Appl Physiol 102:2207–2216
Conraads VM, Jorens PG, De Clerk LS, van Saene HK, Leven MM, Bosmans JM, Schuerwegh A, Bridts CH, Wuyts F, Stevens WJ, Anker SD, Rauchhaus M, Vrints CJ (2004) Selective intestinal decontamination in advanced chronic heart failure: a pilot trial. Eur J Heart Fail 6:483–491
van Saene HKF, Stoutenbeek CP, Faber-Nijholt R, van Saene JJM (1992) Selective decontamination of the digestive tract contributes to the control of disseminated intravascular coagulation in severe liver impairment. J Paediatr Gastroenterol Nutr 14:436–442
van Saene JJM, Stoutenbeek CP, van Saene HKF, Matera G, Martinez-Pellus AE, Ramsay G (1996) Reduction of intestinal endotoxin by three different SDD regimens in human volunteers. J Endotoxin Res 3:337–343
Silvestri L, Rommes JH, Petros AJ, Taylor N, van Saene HKF (2012) Selective decontamination of the digestive tract may reinforce the efficacy of corticosteroids. Am J Respir Crit Care Med 185:344
Silvestri L, Petros AJ, Zandstra DF, Taylor N, van Saene HKF (2011) SDD reduces bacteraemia following eradication of gut overgrowth. Crit Care Med 39:2785–2786
Silvestri L, Zandstra DF, Petros AJ, Taylor N, van Saene HKF (2012) Gravity may be the law, but it does not impact critical illness related carriage in overgrowth. Minerva Anestesiol 78:507–510
Mandelli M, Mosconi P, Langer M, Cigada M and the Intensive Care Unit Group of Infection Control (1989) Prevention of pneumonia in an intensive care unit: a randomised multi-centre clinical trial. Crit Care Med 17:501–505
Stoutenbeek CP (1990) Prevention of pneumonia in an intensive care unit. Crit Care Med 18:1190–1191
Silvestri L, de la Cal MA, Taylor N, van Saene HKF, Parodi PC (2010) Selective decontamination of the digestive tract in burn patients: an evidence based manoeuvre that reduces mortality. J Burn Care Res 31:372–373
Silvestri L, van Saene HKF, Zandstra DF, Viviani M, Gregori D (2010) SDD, SOD or oropharyngeal chlorhexidine to prevent pneumonia and to reduce mortality in ventilated patients: which manoeuvre is evidence based? Intensive Care Med 31:1436–1437
de Smet AM, Kluytmans JA, Blok HE, Mascini EM, Benus RF, Bernards AT, Kuijper EJ, Leverstein-van Hall MA, Jansz AR, de Jongh BM, van Asselt GJ, Frenay IH, Thijsen SF, Conijn SN, Kaan JA, Arends JP, Sturm PD, Bootsma MC, Bonten MJ (2011) Selective digestive tract decontamination and selective oropharyngeal decontamination and antibiotic resistance in patients in intensive-care units: an open-label, clustered group-randomised, crossover study. Lancet Infect Dis 11:372–380
Abecasis F, Sarginson RE, Kerr S, Taylor N, van Saene HKF (2011) Is selective digestive decontamination useful in controlling aerobic gram-negative bacilli producing extended spectrum beta-lactamases? Microb Drug Resist 17:17–23
Saidel-Odes L, Polachek H, Peled N, Riesenberg K, Schlaeffer F, Trabelsi Y, Eskira S, Yousef B, Smolykov R, Codish S, Borer A (2012) A randomized, double-blind, placebo-controlled trial of selective digestive decontamination using oral gentamicin and oral polymyxin E for eradication of carbapenem-resistant Klebsiella pneumoniae carriage. Infect Control Hosp Epidemiol 33:14–19
Laupland KB, Fisman DN (2009) Selective digestive tract decontamination: a tough pill to swallow. Can J Infect Dis Med Microbiol 20:9–11
Silvestri L, van Saene HKF (2006) Selective decontamination of the digestive tract does not increase resistance in critically ill patients: evidence from randomised controlled trials. Crit Care Med 34:2027–2030
Vincent JL, Jacobs F (2011) Effect of selective decontamination on antibiotic resistance. Lancet Infect Dis 11:337–338
Bion J, Taylor N, Petros AJ, Silvestri L, van Saene HKF (2012) Selective digestive decontamination and antibiotic resistance. Lancet Infect Dis 12:181
Wunderink RG (2010) Welkommen to our world: emergence of antibiotic resistance with selective decontamination of the digestive tract. Am J Respir Crit Care Med 181:426–428
Oostdijk EA, de Smet AM, Blok HE, Thieme Groen ES, van Asselt GJ, Benus RF, Bernards SA, Frénay IH, Jansz AR, de Jongh BM, Kaan JA, Leverstein-van Hall MA, Mascini EM, Pauw W, Sturm PD, Thijsen SF, Kluytmans JA, Bonten MJ (2010) Ecological effects of selective decontamination on resistant Gram-negative bacterial colonisation. Am J Respir Crit Care Med 181:452–457
Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, Montori V, Akl EA, Djulbegovic B, Falck-Ytter Y, Norris SL, Williams JW Jr, Atkins D, Meerpohl J, Schünemann HJ (2011) GRADE guidelines: 4 Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol 64:407–415
Petros AJ, Taylor N, Damjanovic V, van Saene HKF, Abecasis F, Silvestri L, de la Cal MA (2010) Worlds apart: proof that SDD works. Am J Respir Crit Care Med 182:1564–1565
Sackett DL (2000) The sins of expertness and a proposal for redemption. BMJ 320:1283
Silvestri L, Petros AJ, de la Cal MA, Visintin S (2011) Selective digestive decontamination. Why are intensivists more ‘resistant’ than micro-organisms? Minerva Anesthesiol 77:658–659
van Saene HKF, Silvestri L, Taylor N, de la Cal MA, Petros A, Zandstra DF (2012) Selective decontamination and ecology. Anaerobe 18:361–362
van Saene HKF, Stoutenbeek CP (1987) Selective decontamination. J Antimicrob Chemother 20:462–465
Ochoa-Ardila ME, García-Cañas A, Gómez-Mediavilla K, González-Torralba A, Alía I, García-Hierro P, Taylor N, van Saene HK, de la Cal MA (2011) Long-term use of selective decontamination of the digestive tract does not increase antibiotic resistance: a 5-year prospective cohort study. Intensive Care Med 37:1458–1465
Paulus SC, van Saene HKF, Hemsworth S, Hughes J, Ng A, Pizer BL (2005) A prospective study of septicaemia on a paediatric oncology unit: a three-year experience at The Royal Liverpool Children’s Hospital, Alder Hey, UK. Eur J Cancer 41:2132–2140
van Saene HK, Silvestri L, Taylor M, Petros A, Bion J (2012) Treatment of sepsis. Lancet Infect Dis 12 (in press)
Acknowledgments
The authors acknowledge the careful preparation of the manuscript by Nia Taylor, who meticulously checked the data from the 65 RCTs and 11 meta-analyses. Dr Derrick Baxby contributed significantly to the first two reviews and is gratefully acknowledged for his careful review of this third update.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silvestri, L., de la Cal, M.A. & van Saene, H.K.F. Selective decontamination of the digestive tract: the mechanism of action is control of gut overgrowth. Intensive Care Med 38, 1738–1750 (2012). https://doi.org/10.1007/s00134-012-2690-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2690-1