Abstract
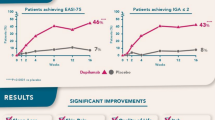
Dupilumab (Dupixent®), a subcutaneously administered, fully human IgG4 monoclonal antibody directed against the IL (interleukin)-4 receptor α subunit, blocks the signalling of IL-4 and IL-13, two T helper cell type 2 cytokines implicated in the immunopathology of atopic dermatitis (AD). It is the first biologic therapy to have been approved for the treatment of adult patients with moderate-to-severe AD in the EU and USA. In phase III trials in adults with moderate-to-severe AD who were inadequately controlled with topical medications and/or systemic treatments, such as ciclosporin, or for whom these therapies were not advisable, 16 weeks’ treatment with dupilumab as monotherapy or in combination with topical corticosteroids (TCS) improved multiple measures of disease severity, pruritus, sleep disturbance, anxiety and depression, and quality of life compared with placebo. Moreover, the benefits of combination therapy at week 16 were maintained during long-term treatment for up to 1 year. Dupilumab, alone or added to TCS, was generally well tolerated, with low rates of serious adverse events and treatment discontinuations due to adverse events. Common adverse reactions included conjunctivitis, injection-site reactions and oral herpes. Thus, dupilumab represents a valuable new treatment option for adults with moderate-to-severe AD deemed appropriate for systemic therapy, a patient population for whom historically there has been a lack of safe and effective long-term treatments.
Similar content being viewed by others
References
Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387(10023):1109–22.
Boguniewicz M, Alexis AF, Beck LA, et al. Expert perspectives on management of moderate-to-severe atopic dermatitis: a multidisciplinary consensus addressing current and emerging therapies. J Allergy Clin Immunol Pract. 2017;5(6):1519–31.
Nygaard U, Vestergaard C, Deleuran M. Emerging treatment options in atopic dermatitis: systemic therapies. Dermatology. 2017;233(5):344–57.
Eckert L, Gupta S, Amand C, et al. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the United States: an analysis using the National Health and Wellness Survey. J Am Acad Dermatol. 2017;77(2):274–279.e3.
Simpson EL, Bieber T, Eckert L, et al. Patient burden of moderate to severe atopic dermatitis (AD): insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol. 2016;74(3):491–8.
Megna M, Napolitano M, Patruno C, et al. Systemic treatment of adult atopic dermatitis: a review. Dermatol Ther (Heidelb). 2017;7:1–23.
Paller AS, Kabashima K, Bieber T. Therapeutic pipeline for atopic dermatitis: end of the drought? J Allergy Clin Immunol. 2017;140(3):633–43.
Renert-Yuval Y. Guttman-Yassky. Monoclonal antibodies for the treatment of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2018;18(4):356–64.
Hamilton JD, Ungar B, Guttman-Yassky E. Drug evaluation review: dupilumab in atopic dermatitis. Immunotherapy. 2015;7(10):1043–58.
EMA. Dupixent 300 mg solution for injection in pre-filled syringe: EU summary of product characteristics. 2017. http://www.ema.europa.eu. Accessed 7 Jul 2018.
US FDA. Dupixent® (dupilumab) injection, for subcutaneous use: US prescribing information. 2017. https://www.fda.gov. Accessed 7 Jul 2018.
Sanofi Co., Ltd. Dupixent: Japanese prescribing information. 2018.
Radin A, Ren H, Papino-Wood P, et al. First-in-human study of REGN668/SAR231893 (IL-4Ra mAb): safety, tolerability and biomarker results of a randomized, double-blind, placebo-controlled, single ascending dose study in healthy volunteers [abstract no. AB158]. J Allergy Clin Immunol. 2013;131(2):AB158.
Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371(2):130–9.
Hamilton JD, Beck BLA, Ren H, et al. Biomarkers elevated in atopic dermatitis (AD) are reduced by therapeutic blockade of IL-4 receptor alpha (IL-4Ralpha) signaling with REGN668/SAR231893 in patients with severe AD [abstract]. J Investig Dermatol. 2013;133:S177.
Hamilton J, Hamon S, Simpson E, et al. The effect of dupilumab on biomarkers in a randomized phase 2b clinical trial in adults with moderate-to-severe atopic dermatitis [abstract no. 372]. J Invest Dermatol. 2016;136(9 Suppl 2):S224.
Guttman-Yassky E, Hamilton J, Bissonnette R, et al. Dupilumab improves clinical atopic dermatitis parameters and modulates specific IgEs and Staphylococcus aureus abundance [abstract no. 373]. J Invest Dermatol. 2016;136(9 Suppl 2):S224.
Hamilton J, Hamon S, Chaudhry U, et al. Dupilumab suppression of Th2 biomarkers correlates with reduction in transepidermal water loss (TEWL) and clinical improvements in adults with moderate-to-severe atopic dermatitis (AD) [abstract no. 169]. J Investig Dermatol. 2015;135(Suppl 1):S29.
Guttman-Yassky E, Suarez-Farinas M, Ungar B, et al. Dupilumab progressively suppresses inflammation, reduces epidermal hyperplasia and increases epidermal barrier gene expression in atopic dermatitis (AD) skin [abstract no. P122]. Exp Dermatol. 2016;25(Suppl 4):47–8.
Hamilton JD, Suarez-Farinas M, Dhingra N, et al. Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol. 2014;134(6):1293–300.
Blauvelt A, Simpson E, Wu R, et al. The effect of dupilumab on vaccine antibody responses in adults with moderate-to-severe atopic dermatitis: a randomized, double-blind, placebo-controlled trial [abstract no. 1347]. Allergy. 2016;71(Suppl 102):95.
Kovalenko P, DiCioccio AT, Davis JD, et al. Exploratory population PK analysis of dupilumab, a fully human monoclonal antibody against IL-4Rα, in atopic dermatitis patients and normal volunteers. CPT Pharm Syst Pharmacol. 2016;5(11):617–24.
Kovalenko P, Davis JD, Li M, et al. Population pharmacokinetic analysis of dupilumab using early phase and phase 3 data [abstract no. T-034]. J Pharmacokinet Pharmacodyn. 2017;44(Suppl 1):S69–70.
Davis JD, Rawal S, Kamal M, et al. Pharmacokinetics of dupilumab in long-term phase III studies in adult patients with moderate-to-severe atopic dermatitis [abstract no. PII-029]. Clin Pharmacol Ther. 2017;101(Suppl 1):S61.
Davis JD, Bansal A, Hassman D, et al. The effect of dupilumab on the pharmacokinetics of cytochrome P450 substrates in adult patients with moderate-to-severe atopic dermatitis: an open label phase 1 trial [abstract no. 1070]. Allergy. 2017;72(Suppl 103):604–5.
Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–48.
Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287–303.
De Bruin-Weller M, Thaci D, Smith CH, et al. Dupilumab with concomitant topical corticosteroids in adult patients with atopic dermatitis who are not adequately controlled with or are intolerant to ciclosporin A, or when this treatment is medically inadvisable: a placebo-controlled, randomized phase 3 clinical trial (LIBERTY AD CAFE). Br J Dermatol. 2018;178(5):1083–101.
De Bruin-Weller MS, Bieber T, Kawashima M, et al. Efficacy and safety of dupilumab in adult patients with atopic dermatitis and an inadequate response, intolerance, or contraindication to cyclosporine: pooled analysis of two 16-week phase 3 trials [abstract no. 0029]. Allergy. 2017;72(Suppl 103):46–7.
De Bruin-Weller MS, Bieber T, Kawashima M, et al. Efficacy and safety of dupilumab in adult patients with atopic dermatitis and an inadequate response, intolerance, or contraindication to cyclosporine: subgroup analysis from a 1-year trial [abstract no. 0030]. Allergy. 2017;72(Suppl 103):47–8.
US National Institutes of Health. 2017. https://clinicaltrials.gov/ct2/show/NCT03054428. Accessed 7 Jul 2018.
Silverberg J, Chao J, Eckert L, et al. Dupilumab treatment rapidly improves itch in patients with moderate-to-severe atopic dermatitis [abstract no. P481]. Ann Allergy Asthma Immunol. 2017;119(Suppl 5):S95.
Simpson EL. Dupilumab improves general health-related quality-of-life in patients with moderate-to-severe atopic dermatitis: pooled results from two randomized, controlled phase 3 clinical trials. Dermatol Ther (Heidelb). 2017;7(2):243–8.
US FDA. Dupixent (dupilumab): medical review. 2016. https://www.accessdata.fda.gov. Accessed 7 Jul 2018.
Moreno AS, McPhee R, Arruda LK, et al. Targeting the T helper 2 inflammatory axis in atopic dermatitis. Int Arch Allergy Immunol. 2016;171:71–80.
Kraft M, Worm M. Dupilumab in the treatment of moderate-to-severe atopic dermatitis. Expert Rev Clin Immunol. 2017;13(4):301–10.
Vangipuram R, Tyring SK. Dupilumab for moderate-to-severe atopic dermatitis. Skin Ther Lett. 2017;22(6):1–4.
Fleming P, Drucker AM. Risk of infection in patients with atopic dermatitis treated with dupilumab: a meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2018;78(1):62–69.E1.
Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. JEADV. 2018;32:850–78.
National Institute for Health and Care Excellence. Dupilumab for treating moderate to severe atopic dermatitis after topical treatments [ID1048]: final appraisal determination document. 2018. https://www.nice.org.uk/guidance/gid-ta10218/documents/final-appraisal-determination-document. Accessed 28 June 2018.
Ariens LFM, Bakker DS, van der Schaft J, et al. Dupilumab in atopic dermatitis: rationale, latest evidence and place in therapy. Ther Adv Chronic Dis. 2018. https://doi.org/10.1177/2040622318773686.
Acknowledgements
During the peer review process, the manufacturer of dupilumab was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
James Frampton and Hannah Blair are salaried employees of Adis/Springer, are responsible for the article content and declare no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: M Gooderham, Department of Medicine, Queen’s University, Kingston, ON, Canada; M. Megna, Department of Dermatology, University of Naples Federico II, Naples, Italy.
Rights and permissions
About this article
Cite this article
Frampton, J.E., Blair, H.A. Dupilumab: A Review in Moderate-to-Severe Atopic Dermatitis. Am J Clin Dermatol 19, 617–624 (2018). https://doi.org/10.1007/s40257-018-0370-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-018-0370-9