Abstract
Pneumococcal polysaccharide conjugate vaccine (13-valent, adsorbed) [PCV13] is approved for protection against pneumococcal disease in children aged 6 weeks to 5 years and adults aged ≥50 years.
In randomized trials in adults aged 60–64 years (not previously vaccinated with 23-valent pneumococcal polysaccharide vaccine [PPV23]) and ≥70 years (previously vaccinated with PPV23), PCV13 was non-inferior to PPV23 in opsonophagocytic assay (OPA) geometric mean titres (GMTs) for all 12 serotypes common to the two vaccines. More PCV13 than PPV23 recipients had ≥4-fold increases in serotype 6A OPA GMTs (serotype 6A is not included in PPV23). PCV13 recipients also had higher OPA GMTs and met superiority criteria for most serotypes.
Adults aged 50–59 years had antibody responses to PCV13 that were noninferior to those in adults aged 60–64 years for all included serotypes. PCV13 administered concomitantly with trivalent inactivated influenza vaccine in adults aged 50–59 or ≥65 years produced antibody responses that were noninferior to those following sequential administration, except for influenza strain A/H3N2 and pneumococcal serotype 19F in those aged ≥65 years. Antibody responses were numerically higher with sequential administration, although the clinical significance of this is unknown.
Adverse events within 14 days of vaccination were mostly of mild-to-moderate severity, with serious events occurring in 0.2–1.4% of PCV13 and 0.4–1.7% of PPV23 recipients.
Similar content being viewed by others

Streptococcus pneumoniae is a human pathogen that causes a variety of diseases, including otitis media, chiefly in children, and invasive pneumococcal disease (IPD) in children and adults.[1] IPD is an important cause of morbidity and mortality, resulting from pneumonia, meningitis or septicaemia/bacteraemia.[1,2] The major site of colonization by S. pneumoniae is the nasopharynx, with transmission of the bacteria from the colonized individual to other contacts.[1] It is thought that IPD results when the bacterium spreads from the nasopharynx to the lower respiratory tract and other sites. There are more than 90 pneumococcal serotypes, which vary in their potential for colonization, virulence and likelihood of causing these diseases. While there is geographical variation in the prevalence of specific serotypes, it is thought that 20 serotypes account for >80% of IPD, globally.[1]
Host factors, such as compromised immunity, are important in the development and clinical course of pneumococcal diseases, with both disease and mortality rates particularly high in young children in developing countries, probably as a result of poverty, malnutrition, poor access to healthcare and co-morbid illnesses.[1] Other established risk factors for IPD and pneumococcal pneumonia include age (high risk for individuals <2 years or ≥65 years), antecedent influenza, defects in humoral immunity, HIV infection, alcoholism, diabetes mellitus, asplenia or hyposplenia, and recent acquisition of a new virulent pneumococcal strain.[3]
There is considerable variability between countries in the incidence of pneumococcal diseases, resulting in part from the different surveillance and diagnostic testing systems in place. In EU countries that report pneumococcal infections, the 2007 notification rate for IPD was 6.0 cases per 100 000 population.[2] In the US, the 2009 estimates for IPD (defined as S. pneumoniae isolated from a normally sterile site) were 12.9 cases and 1.2 deaths per 100 000 population; the case rates were higher in children aged <5 years and adults ≥65 years (18.0 and 37.0 cases per 100 000, respectively).[4] In adults aged ≥65 years in the US, the estimated mortality from invasive disease was 5.6 per 100 000, compared with 1.3 per 100 000 for all ages.[4] From a public health perspective, non-bacteraemic pneumococcal pneumonia is the most important of the adult pneumococcal diseases, as it is much more common than bacteraemic disease, accounting for ≈30% of patients hospitalized with community acquired pneumonia.[5]
The first available vaccines against IPD were unconjugated vaccines consisting of pneumococcal capsular polysaccharide antigens. The most recent formulation is the 23-valent pneumococcal polysaccharide vaccine (Pneumovax 23®) [PPV23], which has been available in the US and elsewhere since the 1980s.[5] PPV23 protects against IPD in some adults, with a vaccine efficacy of ≈60% in observational studies in healthy elderly adults.[5,6] However, PPV23 has a number of limitations. It induces poor vaccine responses in infants <2 years of age,[3] in at least a subset of elderly adults,[7] and in immunocompromised patients.[3] Two recent meta-analyses of PPV23 effects in adult populations concluded that there was no convincing evidence of protection against all-cause pneumonia,[8,9] presumptive pneumococcal pneumonia[9] or all-cause mortality.[8,9] In a well designed trial in Uganda, PPV23 failed to protect adults with HIV-1 infection against IPD.[10] Finally, in children, 14-valent pneumococcal polysaccharide vaccine failed to significantly reduce nasopharyngeal carriage of S. pneumoniae,[11] which would suggest that PPV23 is unlikely to be associated with indirect (herd) protection.
Conjugate vaccines, including those against S. pneumoniae, Haemophilus influenzae and Neisseria meningitidis, were developed to produce an enhanced immunological response.[12] These vaccines contain carrier proteins to which the polysaccharide antigens are joined. The protein antigens provoke immune responses that require T-cell help, resulting in enhanced immunogenicity and priming for memory responses. In contrast, unconjugated vaccines rely for their effects exclusively on capsular polysaccharide antigens, producing an immune response that is limited to relatively short-lived increases in antibodies and that does not lead to booster responses on revaccination. This is especially problematic in infants in whom vaccines containing only capsular polysaccharide fail to produce an effective antibody response.[12]
A vaccine containing seven serotype-specific polysaccharides of S. pneumoniae capsular antigens conjugated individually to non-toxic diphtheria CRM197 protein (Prevenar 7®) [PCV7] was released in 2000.[13] This vaccine includes serotypes that accounted for >60% of pneumococcal infections in children in developed countries at that time.[13] Following the introduction of PCV7, in several countries using the vaccine there was a marked fall in the incidence of pneumococcal pneumonia, bacteraemia and meningitis in children, which was also observed in unvaccinated siblings and adults.[3] These disease reductions in non-vaccinated populations suggest that PCV7 vaccination programmes are associated with indirect immunity.[3] Promising protective effects with PCV7 were also seen in immunocompromised Malawian adults and adolescents with HIV infection recently recovered from pneumococcal disease, as it was protective against further pneumococcal diseases caused by vaccine serotypes and non-vaccine serotype 6A.[14]
Nevertheless, along with declining IPD rates in countries using PCV7 in children, there has been an increase in the prevalence of pneumococcal infections involving serotypes that are not included in the vaccine and evidence that some of these serotypes are virulent and/or antibiotic resistant.[1] This changing pattern of serotype-specific pneumococcal infections is a cause for concern and justifies further vaccine development, including expansion of pneumococcal serotypes in the conjugate vaccine to include those not covered by PCV7.
A 13-valent pneumococcal conjugate vaccine (Prevenar 13®; Prevnar 13®) [PCV13] was developed to meet this need for a vaccine with wider coverage of pneumococcal serotypes.[6] As for PCV7, the individual serotype-specific polysaccharides are conjugated to diphtheria CRM197 protein and include serotypes 1, 3, 5, 6A, 7F and 19A as well as all the serotypes included in PCV7 (4, 6B, 9 V, 14, 18C, 19F and 23F). PCV13 is approved in many countries worldwide for the prevention of IPD in infants and young children aged 6 weeks to 5 years.[13] In children, PCV13 produced robust responses against all 13 pneumococcal serotypes included in the vaccine, with generally similar responses to PCV7 for the serotypes in common, while a second dose boosted the immune response against all 13 serotypes.[13] Furthermore, PCV13 reduced pneumococcal nasopharyngeal carriage in children aged <2 years with acute otitis media[15] and in healthy infants.[16]
PCV13 was recently approved in the US[17] and the EU[18] for use in adults aged ≥50 years for the prevention of pneumonia (US) and invasive disease (US, EU) caused by S. pneumoniae. PCV13 is also approved for the prevention of pneumococcal diseases in adults aged ≥50 years in several other countries, worldwide.[19] Both the US FDA and the European Medicines Agency approved PCV13 for use in adults on the basis that it met the licensing criterion of noninferiority of immune (humoral) responses to PPV23. This article reviews immunogenicity studies of PCV13 in adults, along with relevant information regarding its tolerability and likely cost effectiveness. Medical literature (including published and unpublished data) on the use of PCV13 in preventing IPD in older adults was identified by searching databases (including MEDLINE and EMBASE) for articles published since 1996, bibliographies from published literature, clinical trial registries/databases and websites (including those of regional regulatory agencies and the manufacturer). Additional information (including contributory unpublished data) was also requested from the company developing the vaccine. Searches were last updated 25 May 2012.
1. Immunogenicity
The immunogenicity of PCV13 (antibody responses to vaccine antigens) has been evaluated in PPV23-naive adults aged 50–64 years (study 1),[17,18,23] in adults aged ≥70 years who had received a single dose of PPV23 at least 5 years previously (study 2),[17,18,20] and in PPV23-naive adults aged 60–64 years who received an initial dose of PCV13 or PPV23 (PCV13 recipients received PPV23 1 year later and PPV23 recipients received PCV13 1 year later) [study 3].[17] Further studies evaluated the immunogenicity of PCV13 and the trivalent inactivated influenza vaccine (TIV) administered concomitantly versus after sequential administration (TIV followed 1 month later by PCV13) [studies 5 and 6].[17,18,21] Study 5 is discussed only in brief, as its design was the same as that of study 6, except that the age of the population enrolled differed (50–59 years in study 5 and ≥65 years in study 6) and the results of the two studies were generally consistent.[21] Data for studies 1–3 and 5 are available from abstracts[20,23] and/or the manufacturer’s prescribing information.[17,18] Study 6 is fully published.[21] Study 4 was a phase II immunogenicity study and is not discussed further in this review. The key inclusion criteria, primary study objectives and treatment groups after randomization in cited studies are shown in table I.
The studies were all randomized, multicentre trials with vaccines either administered in a double-blind (studies 5 and 6)[17,18,20,21] or modified double-blind (studies 1, 2 and 3)[17,18,23] fashion; in study 1, the cohort aged 50–59 years received open-label immunization with PCV13.[17] In the modified double-blind condition, both investigators and participants were blinded to the vaccine, whereas staff administering the vaccine were not blinded.[17]
Participants in studies 1–3, 5 and 6 were healthy adults, or immunocompetent adults with chronic stable medical conditions, who were PPV23 vaccine-naive (studies 1, 3, 5 and 6) or vaccinated with PPV23 at least 5 years earlier (study 2).[20,21,23]
In all studies, the main serology endpoints were evaluated 1 month after vaccination.[17,18,20,21,23] Endpoints based on immune responses to vaccine were chosen on the grounds that PPV23 induces a protective immune response against IPD; hence noninferiority to PPV23 would be demonstrated for the immune responses to the 12 serotypes in common and 6A. The PPV23 vaccine contains 25 μg of polysaccharide for each of the serotypes,[24] whereas PCV13 contains 2.2 μg, except for 6A, which contains 4.4 μg,[18] reflecting the higher immunogenicity of conjugate vaccines.
Studies 1 and 2 were noninferiority trials in adults aged 60–64 years[17,18,23] and ≥70 years.[17,18,20] The coprimary endpoints were (i) the serotype-specific opsonophagocytic assay (OPA) geometric mean titres (GMTs) for 12 pneumococcal serotypes common to the two vaccines (noninferiority was shown if the lower limit of the 95% CI for the geometric mean ratio (GMR) [GMT PCV13 : GMT PPV23]) was >0.50); and (ii) the proportion of participants with a ≥4-fold increase in the OPA GMT for serotype 6A (superiority was shown if the lower bound of the 95% CI for the between-group difference in proportions was >0).[17,18,20,23] For the cohort aged 50–59 years in study 1, the primary objective was to demonstrate noninferiority of the immune response in this age group to that in the 60–64 year age group for all 13 serotypes (noninferiority required that the lower limit of the 95% CI for the GMR [GMT age 50–59 years : GMT age 60–64 years] was >0.5).[17] Two-sided tests were used in the primary statistical analyses.[17,18,20,23]
In study 3, a key objective was to evaluate whether the immune response to PPV23 administered 1 year after an initial study dose of PCV13 was noninferior to an initial study dose of PPV23 for the 12 serotypes in common, as measured by serotype-specific OPA titres obtained 1 month after vaccination (noninferiority required that the lower limit of the 95% CI for the GMR [GMT PPV23 after PCV13 : GMT PPV23 as initial dose] was >0.5).[17,22] Further objectives were to evaluate whether an initial dose of PCV13 was significantly more immunogenic than PCV13 administered 1 year after an initial dose of PPV23 for at least some of the 12 serotypes in common and whether PPV23 administered 1 year after PCV13 was significantly more immunogenic than an initial study dose of PPV23 for at least some of the 12 serotypes in common.[17,22]
In studies 5 and 6, the coprimary endpoints were (i) the proportion of participants with a ≥4-fold increase in haemagglutination inhibition assays (HIA) for the three specific TIV strains (noninferiority was shown if the lower limit of the 95% CI for the between-group difference in proportions was >−10%); and (ii) enzyme-linked immunosorbent assay (ELISA) serotype-specific anticapsular polysaccharide IgG geometric mean concentrations (GMCs) for 13 pneumococcal serotypes (noninferiority was shown if the lower limit of the 95% CI for the GMC ratio [concomitant : sequential] was >0.50).[17,21] For influenza vaccines, the European Medicines Association (EMA) vaccine guidelines require that ≥30% of vaccinees respond with ≥4-fold increases in serotype-specific HIA titres to indicate that the vaccine provides a satisfactory protective effect against influenza, and that ≥60% of vaccinees achieve titres ≥40.[21] These criteria were also applied in study 6.[21] The FDA guidelines for efficacy of influenza vaccines relevant to study 5 indicate that appropriate endpoints could be the percentage of patients with an HIA titre of ≥1 : 40 and/or the percentage of patients with an HIA titre <1 : 10 who achieve a titre of 1 : 40, or have a ≥4-fold rise in titres, following vaccination.[25]
In 23-Valent Pneumococcal Polysaccharide Vaccine (PPV23)-Naive Adults
-
In study 1, when evaluated 1 month after vaccination, PCV13 was noninferior to PPV23 for all 12 serotypes common to the vaccines, based on the GMR (PCV13 : PPV23) [coprimary endpoint] (table II).[17,18,23]
-
PCV13 was associated with significantly greater immune responses than PPV23 for 8 of 12 serotypes common to the vaccines, as the lower limit of the 95% CI for the GMR (PCV13 : PPV23) was >1 for each of these serotypes (prespecified superiority criterion) [table II].[17,18,23]
-
For serotype 6A (which is not included in the PPV23 vaccine), a higher proportion of PCV13 recipients had a ≥4-fold increase in the OPA GMT than PPV23 recipients (88.5% vs 49.3%; difference 39.2 [95% CI 33.0, 45.1]) [coprimary endpoint].[17,18,23]
-
Furthermore, the OPA GMT for serotype 6A was higher in the PCV13 group than the PPV23 group, as the lower limit of the 95% CI for the GMR (PCV13 : PPV23) was >2 (prespecified secondary endpoint) [table II].[17,18]
-
The immune response in PPV23-naive adults aged 50–59 years was noninferior to that in adults aged 60–64 years, as the noninferiority criterion was met for all 13 pneumococcal serotypes [table II].[17,18,23]
-
Based on a superiority criterion of a lower limit of the 95% CI for the GMR (age 50–59 years : age 60–64 years) of >1, the immune response was greater in the participants aged 50–59 years than in participants aged 60–64 years for 9 of 13 serotypes (table II).[17,18,23]
-
In adults aged 50–59 years and 60–64 years, OPA GMTs had declined by 1 year after vaccination, although, for each serotype, they remained numerically higher than baseline levels (statistical analyses not reported).[18]
-
In study 3, PCV13 did not diminish the response to subsequent PPV23, as immune responses for the 12 serotypes in common in participants who received PPV23 after PCV13 were noninferior to those following a single dose of PPV23 (groups A and B in table III).[17] For six of the serotypes in common, the immune response was greater in participants who received PPV23 after PCV13 (superiority required a lower limit of the 95% CI for the GMR of >1.0). When PCV13 was administered 1 year after PPV23 versus initial vaccination with PCV13, the immune response was lower for all 12 serotypes common to the two vaccines (upper limit of the 95% CI of the GMR of <1.0) [groups C and D in table III].[17,22] Based on these results, if both PCV13 and PPV23 are to be used, PCV13 should be given first.
Immunogenicity of PCV13 in adults aged ≥50 years. GMTs,a GMCsb and GMRs in randomized, multicentre trials in pneumococcal vaccine-naive adults (studies 1[17,18,23] and 6[17,18,21]) and adults previously vaccinated with PPV23 (study 2) are shown.[17,18,20] Participant numbers analysed by study group were as follows: study 1, age group 60–64 y (PCV13 359–404, PPV23 367–402) and age group 50–59 y (PCV13 350–384); study 2 (PCV13 400–426, PPV23 395–445); study 6 (concomitant 247–279, sequential 247–278)
Immunogenicity of PCV13 and PPV23 in PPV23-naive adults aged 60–64 years after sequential administration of the two vaccines (study 3).[17,22] Participants were randomized to receive either PCV13 as the initial vaccine followed 1 year later by PPV23, or PPV23 as the initial vaccine followed 1 year later by PCV13. The data shown are opsonophagocytic assay GMTs (95% confidence intervals) for serotypes common to the vaccines and serotype 6A and the GMRs for the comparator groups. Participant numbers are shown in square parentheses
In Adults Previously Vaccinated with PPV23
-
In study 2 in adults aged ≥70 years vaccinated with PPV23 at least 5 years previously, the immunogenicity of PCV13 was noninferior to that of PPV23, as the noninferiority criterion was met for all 12 serotypes common to the two vaccines [table II].[17,18,20] For 10 of these 12 serotypes, PCV13 met the prespecified criterion for superiority over PPV23 (lower limit of the 95% CI for the GMR of >1) [table II].[17,18,20]
-
For serotype 6A, a higher proportion of PCV13 recipients had a ≥4-fold increase in the OPA GMT than PPV23 recipients (71.1% vs 27.3%; difference 43.8 [95% CI 37.4, 49.9]) [coprimary endpoint].[17,18,20]
-
The OPA GMT for serotype 6A was also higher in the PCV13 group than the PPV23 group, with a lower limit of the 95% CI for the GMR (PCV13 : PPV23) of >2 (prespecified secondary endpoint) [table II].[17,18]
-
As for the younger age group, in adults aged ≥70 years, OPA GMTs declined during the first year after vaccination, but remained numerically higher than baseline levels for each serotype (statistical analyses not reported).[18]
Administered with Trivalent Inactivated Influenza Vaccine
-
In study 6, in adults aged ≥65 years, the concomitant administration group was noninferior to the sequential group in antibody response to two of three TIV antigens.[21] For serotypes A/H1N1 and B, the concomitant administration group met the noninferiority criterion, whereas for serotype A/H3N2, the lower limit of the 95% CI was −10.4%, which just exceeded the prespecified limit of −10% (table IV).[21]
-
Both the concomitant and the sequential administration groups met EMA vaccine guideline criteria for all three TIV serotypes (table IV).[21]
-
For all pneumococcal serotypes except for 19F, concomitant administration of PCV13 with TIV was noninferior to sequential administration, based on GMC ratios derived from ELISA estimates (table II).[21] However, for all 13 serotypes, the GMC was numerically higher in the sequential administration group (table II). In the absence of an established correlate of protection based on the GMC, the clinical significance of this is unknown.[21]
-
In study 5, which was conducted in adults aged 50–59 years, concomitant administration of PCV13 with TIV produced antibody responses that were noninferior to sequential administration for each of the three TIV strains.[17] Concomitant administration was also noninferior to sequential administration in terms of IgG responses for all 13 serotypes (based on ELISA); in post hoc analyses, a noninferiority criterion based on OPA GMC ratios was met for 8 of 13 serotypes.[17]
Immune responses to PCV13 administered concomitantly or sequentially with TIV in a randomized, double-blind, placebo-controlled study.[21] Participants were randomized to TIV plus PCV13 followed by placebo at 1 month (concomitant) or TIV plus placebo followed by PCV13 at 1 month (sequential). Percentages of participants with a ≥4-fold increase from prevaccination baseline in serotype-specific HIA titres for TIV antigens 1 month after the TIV vaccination are shown. The proportions of participants who reached an HIA titre ≥40 are shown in square parentheses
2. Tolerability
The tolerability of PCV13 was evaluated in the immunogenicity studies described in section 1; the focus in this section is on adults aged 60–64 years (study 1)[17,23] and ≥70 years (study 2),[17,20] as these include comparative data for the PPV23 vaccine. Data from study 6 comparing concomitant versus sequential administration of PCV13 and TIV in adults ≥65 years are also discussed.[21] The tolerability of PCV13 in adults aged 50–59 years is not discussed specifically, as it was generally similar to that in the older age groups. All data are from the US prescribing information[17] or study 6,[21] as these sources provide the most detail.
-
Across six immunogenicity trials in adults aged ≥50 years, the most frequent treatment-emergent adverse events occurring within 14 days of vaccination with PCV13 in >5% of patients were pain at the injection site, fatigue, headache, muscle pain, joint pain, reduced appetite, injection site redness, injection site swelling, limitation of arm movement, chills and rash.[17]
-
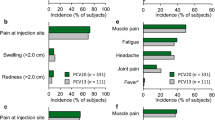
Systemic adverse events of any grade of severity occurring within 14 days of vaccination in ≥1% of PCV13 or PPV23 recipients aged 60–64 years or ≥70 years are shown in figure 1.[17] Events arising within 14 days of vaccination were generally similar between age groups and between the PCV13 and PPV23 groups (figure 1).[17] However, PCV13 recipients aged ≥70 years had significantly (p < 0.05) lower rates of new muscle pain, fatigue and rash than PPV23 recipients (figure 1).[17] The differences in rates were small, and statistical correction for multiple testing was not applied, so the clinical relevance of these between-group differences is uncertain.
-
Local reactions of any severity occurring within 14 days of vaccination are shown in figure 2. In PCV13 and PPV23 recipients across age groups, injection-site pain was the most frequent local reaction to vaccination, occurring in >50% of vaccinees (figure 2).[17] Limitation of arm movement, injection-site redness or swelling occurred in 10–31% of participants across treatment groups and were significantly (p < 0.05) less common in PCV13 than PPV23 recipients in the ≥70 years age group (figure 2) [statistical corrections for multiple tests were not applied].[17]
-
In adults aged ≥50 years, most adverse events were of mild-to-moderate severity.[17] Serious adverse events within 1 month of initial vaccination occurred in 0.2–1.4% of PCV13 recipients and 0.4–1.7% of PPV23 recipients. During the period 1–6 months following initial vaccination, serious adverse events occurred in 1.2–5.8% and 2.4–5.5% in the respective groups. One individual developed erythema multiforme 34 days after a second dose of PCV13.[17]
-
Across trials, 12 of 5667 (0.21%) PCV13 recipients and 4 of 1391 (0.28%) PPV23 recipients died, with all deaths occurring within the period from day 3 to day 309 following vaccination.[17] Two of 12 deaths in PCV13 recipients occurred within 30 days of vaccination (one from cardiac failure and one from peritonitis). Other causes of death (occurring >30 days after vaccination with PCV13) were cardiac disorders and neoplasms (both associated with four deaths), and Mycobacterium avium complex pulmonary infection and septic shock.[17]
-
In adults aged ≥65 years, coadministration of PCV13 with TIV was associated with a higher rate of systemic adverse events than sequential administration of the vaccines (PCV13 given alone 1 month after the initial vaccination with TIV plus placebo).[21] Systemic adverse events occurred in 60.1% of recipients within 14 days of concomitant vaccination with PCV13 and TIV (vs 48.5% after sequential administration; difference 11.6 [95% CI 5.4, 17.8]).[21] Specific adverse events that were more frequent with concomitant administration than with sequential administration were fatigue (37.4% vs 28.5%; difference 8.9 [95% CI 2.8, 14.9]), headache (32.6 vs 24.7; difference 7.9 [95% CI 2.1, 13.8]), aggravated joint pain (15.7 vs 8.6; difference 7.1 [95% CI 2.7, 11.4]), reduced appetite (16.9 vs 11.3; difference 5.6 [95% CI 1.0, 10.2]), chills (13.8 vs 9.1; difference 4.7 [95% CI 0.4, 9.0]) and new joint pain (16.2 vs 11.5; difference 4.7 [95% CI 0.1, 9.2]) [statistical corrections for multiple tests were not applied].[21]
-
Concomitant and sequential administration of the PCV13 and TIV vaccines were associated with generally similar rates of local reactions; any local reaction occurred in 46.9% of recipients of concomitant administration (versus 46.6% with sequential administration; difference 0.3 [95% CI −6.0, 6.7]).[21] The majority of these reactions were mild.[21]
-
Two participants died as a result of serious adverse events (cardiac failure 3 days following vaccination in the concomitant administration group and by gastrointestinal haemorrhage 29 days after vaccination in the sequential administration group).[21] One participant in the concomitant administration group withdrew from the study because of a serious adverse event occurring 10 days after vaccination (angina pectoris with ST-segment elevation). There were no reports of vaccine-related serious adverse events during the course of this study.[21]
General tolerability of PCV13 in older adults. Incidence of systemic adverse events at any grade of severity occurring in ≥1% of PCV13 recipients within 14 days of vaccination in (a) adults aged 60–64 years and (b) adults aged ≥70 years are shown. Data are from the US prescribing information[17] and are derived from studies 1 and 2 (see section 1 for study details). PCV13 = pneumococcal polysaccharide conjugate vaccine (13-valent, adsorbed); PPV23 = 23-valent pneumococcal polysaccharide vaccine; * p < 0.05 (not adjusted for multiple tests).
Local reactions to PCV13 in older adults. Incidence of local reactions at any grade of severity occurring within 14 days of vaccination in (a) adults aged 60–64 years and (b) adults ≥70 years are shown. Data are from the US prescribing information[17] and are derived from studies 1 and 2 (see section 1 for study details). PCV13 = pneumococcal polysaccharide conjugate vaccine (13-valent, adsorbed); PPV23 = 23-valent pneumococcal polysaccharide vaccine; * p < 0.05 (not adjusted for multiple tests).
3. Pharmacoeconomic Considerations
This section discusses findings from a fully published pharmacoeconomic analysis from the Netherlands.[26]
The study used a general decision-tree analytic model to estimate the incremental cost-effectiveness ratio (ICER) for PCV13 administered as a single dose to a hypothetical cohort of adults aged ≥65 years compared with no vaccine.[26] Analyses were from a societal perspective for the total population, and for those at increased risk for pneumonia, and were based on assumptions of PCV13 effectiveness varying from 30–90% (assuming no vaccine effectiveness beyond 5 years). Some models included indirect protection and serotype-replacement effects (effects resulting from increased prevalence of serotypes not included in the vaccine). Estimates of the protective effect of PPV23 were not included in the model, because of restricted use of PPV23 in the Netherlands and uncertainties regarding its effectiveness. Sensitivity analyses were performed to evaluate the robustness of the models.[26]
In general, assumptions about hospitalizations, morbidity and mortality associated with IPD and community-acquired pneumonia were based on national epidemiological and hospital-based study data.[26] Estimates of life-years gained (LYGs) were based on expected variations from predicted life expectancies resulting from pneumococcal-related diseases. Quality-adusted life-years (QALYs) were based on published cost-utility data for older adults. Direct medical costs and indirect costs associated with loss of productivity were based on a 2008 year of costing. Cost and benefit discount rates were 4% and 1.5% annually, respectively.[26]
After taking into account ICER thresholds for the Netherlands of >€80 000/QALY (poor cost effectiveness) and ≤€20 000/LYG [definitely favourable cost effectiveness], a threshold of €50 000/QALY was thought to be a reasonable cost-effectiveness threshold.[26]
-
From a societal perspective in the Netherlands, vaccination of all adults ≥65 years of age was a cost-effective strategy.[26] In the base-case (assuming 5 years of protection, vaccine efficacy of 60% and vaccine costs of €50 per dose), the ICERs were €14 416/QALY for the total population and €8547/QALY for the high-risk population. When indirect effects were included in the models, the ICERs for the corresponding populations were €31 055/QALY and €22 152/QALY.[26]
-
Across models, the ICERs were generally less than €50 000/LYG. For instance, assuming 60% vaccine efficacy against non-invasive pneumococcal disease and IPD and a vaccine cost of €50 per person, the ICERs ranged from cost saving to €50 676/LYG. ICERs were less than €80 000/LYG in all models, except in a conservative model that included indirect effects and assumed a vaccine effectiveness of 40% restricted to bacteraemic pneumonia and a vaccine price of €65.[26]
-
In sensitivity analyses, vaccine efficacy, vaccine price, disease incidence and mortality rate had the largest impact on the ICER.[26] Reduced vaccine cost, which might be possible with mass production of vaccine and by combined use with TIV, appeared likely to have a substantial impact in reducing the ICER.[26]
4. Dosage and Administration
PCV13 is available for the protection of infants and children aged 6 weeks to 5 years and adults aged ≥50 years against pneumococcal infections.[17,18] In adults aged ≥50 years, PCV13 is administered as a single 0.5 mL intramuscular injection, preferably in the deltoid muscle. It should not be injected in the gluteal area, because of the risk of nerve trunk injury or injection into a blood vessel.[17,18]
PCV13 is contraindicated in any patient who has had a severe allergic reaction, such as anaphylaxis, to any component of the vaccine or to any diphtheria toxoid-containing vaccine.[17,18]
There are no available data on PCV13 in patients who are immunocompromised (e.g. patients with HIV infection, congenital or acquired splenic dysfunction, nephrotic syndrome or malignancy and haematopoietic stem cell transplant recipients).[17,18] Patients with these conditions may have a diminished humoral response to the vaccine, as a result of reduced immunity.[17,18]
Local prescribing information should be consulted for details about warnings and precautions, adverse effects and use in special populations.
5. Pneumococcal Polysaccharide Conjugate Vaccine (13-Valent, Adsorbed) in Older Adults: Current Status
PCV13 is approved in many countries worldwide for the prevention of pneumococcal diseases in children.[13] It is also approved in the US[17] and EU[18] for use in adults aged ≥50 years for the prevention of pneumonia (US) and invasive disease (US, EU) caused by S. pneumoniae. PCV13 is approved for the prevention of pneumococcal diseases in adults aged ≥50 years in several other countries, worldwide.[19]
In immunocompetent, pneumococcal vaccine-naive adults aged 60–64 years and previously vaccinated adults aged ≥70 years, the immune response provoked by PCV13 was noninferior to that of the PPV23 vaccine. PCV13 provoked a stronger immune response than PPV23 for the majority of serotypes in common to the vaccines, and for serotype 6A. Immune responses to PCV13 in adults aged 50–59 years were noninferior to those in adults aged 60–64 years. Immune responses to PCV13 and PPV23 administered in different sequences indicate that, if both vaccines are to be used, PCV13 should be administered first. Based on immunogenicity findings from a randomized trial, PCV13 can be administered concomitantly or after vaccination with TIV. Across trials, adverse events with PCV13 were generally of mild-to-moderate severity. Concomitant administration of PCV13 with TIV was associated with a higher incidence of systemic adverse events than sequential administration of the vaccines.
The efficacy of PCV13 in preventing a first episode of community-acquired pneumonia is under evaluation in the CAPiTA (Community Acquired Pneumonia immunization Trial in Adults) trial.[27,28] This trial will be key to clarifying the role that PCV13 can play in preventing pneumococcal pneumonia.
Acknowledgements and Disclosures
The manuscript was reviewed by: S. Antoniu, Pulmonary Disease University Hospital, University of Medicine and Pharmacy Grigore T. Popa Iasi, Iasi, Romania; C. Chouaid, Service de Pneumologie, Hôpital Saint Antoine, Paris, France; S. Sambhara, Influenza Division, Centers for Disease Control and Prevention, Atlanta, GA, USA; P.M. Tulkens, Louvain Drug Research Institute, Université catholique de Louvain, Brussels, Belgium; M. Zeitlinger, Clinical Pharmacology, Medical University of Vienna, Vienna, Austria.
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the author on the basis of scientific and editorial merit.
References
Lynch JP III, Zhanel GC. Streptococcus pneumoniae: epidemiology and risk factors, evolution of antimicrobial resistance, and impact of vaccines. Curr Opin Pulm Med 2010 May; 16 (3): 217–25
European Centre for Disease Prevention and Control. Annual epidemiological report on communicable diseases in Europe 2009 [online]. Available from URL: http://www.ecdc.europa.eu/en/Publications/0910_SUR_Annual_Epidemiological_Report_on_Communicable_Diseases_in_Europe.pdf [Accessed 2011 Nov 17]
van der Poll T, Opal SM. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet 2009 Oct 31; 374 (9700): 1543–56
Centers for Disease Control and Prevention. 2011. Active Bacterial Core Surveillance (ABCs) Report Emerging Infections Program Network Streptococcus pneumoniae, provisional, 2010 [online]. Available from URL: http://www.cdc.gov/abcs/reports-findings/survreports/spneu10.pdf. [Accessed 2012 Mar 14]
Metersky ML, Dransfield MT, Jackson LA. Determining the optimal pneumococcal vaccination strategy for adults: is there a role for the pneumococcal conjugate vaccine?. Chest 2010 Sep; 138 (3): 486–90
Jackson LA, Janoff EN. Pneumococcal vaccination of elderly adults: new paradigms for protection. Clin Infect Dis 2008 Nov 15; 47: 1328–38
Conaty S, Watson L, Dinnes J, et al. The effectiveness of pneumococcal polysaccharide vaccines in adults: a systematic review of observational studies and comparison with results from randomised controlled trials. Vaccine 2004; 22 (23–24): 3214–24
Moberly SA, Holden J, Tatham DP, et al. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2008 Jan 23; (1): CD000422
Huss A, Scott P, Stuck AE, et al. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ 2009 Jan 6; 180 (1): 48–58
French N, Nakiyingi J, Carpenter LM, et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomized and placebo-controlled trial. Lancet 2000 Jun 17; 355: 2106–11
Herva E, Luotenen J, Timonen M, et al. The effect of polyvalent pneumococcal polysaccharide vaccine on nasophar-yngeal and nasal carriage of streptococcus pneumoniae. Scand J Inf Dis 1980; 12 (2): 97–100
Goldblatt D. Conjugate vaccines. Clin Exp Immunol 2000 Jan; 119 (1): 1–3
Duggan ST. Pneumococcal polysaccharide conjugate vaccine (13-valent, adsorbed) [Prevenar 13®]. Drugs 2010; 70 (15): 1973–86
French N, Gordon SB, Mwalukomo T, et al. A trial of a 7-valent pneumococcal vaccine in HIV-infected adults. N Engl J Med 2010 Mar 4; 362 (9): 812–22
Cohen R, Levy C, Bingen E, et al. Impact of 13-valent pneumococcal conjugate vaccine on pneumococcal nasopharyngeal carriage in children with acute otitis media. Pediatr Infect Dis J 2012 Mar; 31 (3): 297–301
Dagan R, Patterson S, Juergens C, et al. Efficacy of 13-valent and 7-valent pneumococcal conjugate vaccines (PCV13; PCV7) in preventing nasopharyngeal colonization with PCV7 serotypes: a randomized, double-blind trial [abstract]. 8th International Symposium on Pneumococci and Pneumococcal Diseases; 2012 Mar 11–15; Iguacu Falls
Pfizer. Prevenar® 13: US prescribing information [online]. Available from URL: http://labeling.pfizer.com/ShowLabeling.aspx?id=501 [Accessed 2012 Apr 3]
European Medicines Agency. Prevenar 13: summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001104/WC500057247.pdf [Accessed 2012 Mar 3]
Pfizer. Pfizer receives FDA approval to extend use of Prevnar 13 for prevention of pneumococcal pneumonia and invasive disease in adults 50 years and older [online]. Available from URL: http://www.pfizer.com/news/ [Accessed 2012 Jan 24]
Jackson L, Gurtman A, Rice K, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine [abstract no. O 425]. 21st European Congress of Clinical Microbiology and Infectious Diseases; 2011 May 7–10; Milan
Schwarz TF, Flamaing J, Rümke HC, et al. A randomized, double-blind trial to evaluate immunogenicity and safety of 13-valent pneumococcal conjugate vaccine given concomitantly with trivalent influenza vaccine in adults aged ≥65 years. Vaccine 2011; 29 (32): 5195–202
Greenberg RN, Gurtmann A, Frenck R, et al. A phase 3, randomized, active-controlled trial to evaluate the safety, tolerability, and immunogenicity of sequential administration of 13-valent pneumococcal conjugate vaccine (PCV13) and 23-valent pneumococcal polysaccharide vaccine (PPSV23) administered at 1-year intervals in PPSV23-naive adults 60–64 years of age [abstract no. 665 plus poster). 49th Annual Meeting of the Infectious Diseases Society of America; 2011 Oct 20–23; Boston (MA)
Jackson L, Gurtman A, van Cleef K, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in pneumococcal vaccine naive adults, 50–64 years of age [abstract no. O 426]. 21st European Congress of Clinical Microbiology and Infectious Diseases; 2011 May 7–10; Milan
Merck and Co., Inc. Pneumovax® 23 (pneumococcal vaccine polyvalent): US precribing information [online]. Available from URL: http://merck.com/product/usa/pi_circulars/p/pneumovax_23/pneumovax_pi.pdf [Accessed 2012 Mar 19]
US Food and Drug Administration. Guidance for industry: clinical data needed to support the licensure of pandemic influenza vaccines [online]. Available from URL: http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm091985.pdf [Accessed 2012 Mar 19]
Rozenbaum MH, Hak E, van der Werf TS, et al. Results of a cohort model analysis of the cost-effectiveness of routine immunization with 13-valent pneumococcal conjugate vaccine of those aged ≥65 years in the Netherlands. Clin Ther 2010; 32 (8): 1517–32
Hak E, Grobbee DE, Sanders EAM, et al. Rationale and design of CAPITA: a RCT of 13-valent conjugated pneumococcal vaccine efficacy among older adults. Neth J Med 2008; 66 (9): 378–83
Study evaluating a 13-valent pneumococcal conjugate vaccine (13vPnC) in adults (CAPITA) [ClinicalTrials.gov identifier NCT00744263]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2012 Jan 23]
Author information
Authors and Affiliations
Corresponding author
Additional information
An Erratum for this chapter can be found at http://dx.doi.org/10.2165/11642220-000000000-00000
Rights and permissions
About this article
Cite this article
Sanford, M. Pneumococcal Polysaccharide Conjugate Vaccine (13-Valent, Adsorbed). Drugs 72, 1243–1255 (2012). https://doi.org/10.2165/11209330-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11209330-000000000-00000