-
PDF
- Split View
-
Views
-
Cite
Cite
Kristine E Ensrud, John L Stock, Elizabeth Barrett‐Connor, Deborah Grady, Lori Mosca, Kay‐Tee Khaw, Qingwen Zhao, Donato Agnusdei, Jane A Cauley, Effects of Raloxifene on Fracture Risk in Postmenopausal Women: The Raloxifene Use for The Heart Trial, Journal of Bone and Mineral Research, Volume 23, Issue 1, 1 January 2008, Pages 112–120, https://doi.org/10.1359/jbmr.070904
Close - Share Icon Share
Abstract
Using data from a randomized placebo‐controlled trial of 10,101 postmenopausal women not selected on the basis of osteoporosis, we examined whether the effect of raloxifene treatment on fractures was consistent across categories of fracture risk. Treatment with raloxifene for 5 yr reduced the risk of clinical vertebral fractures, but not nonvertebral fractures, irrespective of the presence or absence of risk factors for fracture.
Introduction: In The Raloxifene Use for The Heart (RUTH) trial, women assigned to raloxifene had a lower risk of clinical vertebral fractures but not nonvertebral fractures. However, it is uncertain whether the effect of raloxifene on fractures in this population not selected for low BMD differs according to risk factors for fractures.
Materials and Methods: We randomly assigned 10,101 postmenopausal women ≥55 yr of age with documented coronary heart disease or at high risk for coronary events to 60 mg raloxifene daily or placebo and followed them for a median of 5.6 yr. Fractures (nonvertebral and clinical vertebral) were prespecified secondary endpoints that were reported at semiannual visits. Fractures were adjudicated and confirmed using X‐ray reports or medical records.
Results: There was no difference between raloxifene and placebo groups in risk of nonvertebral fractures (428 versus 438 events; hazard ratio [HR], 0.96; 95% CI, 0.84‐1.10), including hip/femur (89 versus 103 events; HR, 0.85; 95% CI, 0.64‐1.13) and wrist (107 versus 111 events; HR, 0.95; 95% CI, 0.73‐1.24) fractures. Women treated with raloxifene had a lower risk of clinical vertebral fractures (64 versus 97 events; HR, 0.65; 95% CI, 0.47‐0.89). The effect of treatment with raloxifene on risk of nonvertebral and clinical vertebral fractures was consistent across fracture risk categories defined at baseline by age, smoking status, physical activity level, prior history of fracture, family history of hip fracture, diabetes mellitus, previous use of hormone therapy, thyroid hormone use, statin use, weight loss, body mass index, or fracture specific summary risk score.
Conclusions: In older women with or at high risk of coronary heart disease not selected on the basis of osteoporosis or increased fracture risk, treatment with raloxifene for 5 yr reduced the risk of clinical vertebral fractures, but not nonvertebral fractures, irrespective of presence or absence of risk factors for fracture.
INTRODUCTION
Raloxifene is a nonsteroidal selective estrogen receptor modulator (SERM) that binds to the estrogen receptor, leading to estrogen‐agonist effects in bone tissue, including an increase in BMD and a reduction in markers of bone turnover.(1) The Multiple Outcomes of Raloxifene Evaluation (MORE) trial that enrolled postmenopausal women with osteoporosis showed that treatment with raloxifene for 3 yr increased BMD at the hip and spine and reduced the risk of radiographic and clinical vertebral fractures but not nonvertebral fractures.(2) However, until the results of The Raloxifene Use for The Heart (RUTH) trial were recently reported,(3) the effect of raloxifene treatment on risk of fracture among postmenopausal women not selected for osteoporosis or increased fracture risk was uncertain.
RUTH was a randomized, double‐blind, placebo‐controlled trial primarily designed to determine the effect of raloxifene on the incidence of coronary events and invasive breast cancer in 10,101 postmenopausal women with or at high risk for coronary heart disease (CHD). As previously reported,(3) treatment with raloxifene for a median of 5.6 yr reduced the risk of invasive breast cancer and clinical vertebral fractures; had no effect on the incidence of coronary events, total strokes, overall mortality, and nonvertebral fractures; and increased the risk of venous thromboembolism and fatal stroke. In this analysis, we evaluated whether the effect of raloxifene on risk of nonvertebral and clinical vertebral fractures was consistent across different categories of fracture risk and determined the effect of raloxifene treatment on risk of hip/femur and wrist fractures.
MATERIALS AND METHODS
Overview
RUTH was a global, multicenter, randomized, double‐blind, placebo‐controlled trial. The study design and methods(3‐5) and the main results of the trial(3) have been reported elsewhere. The two primary objectives of RUTH were to determine the effects of raloxifene on the incidence of coronary events and invasive breast cancer. An a priori secondary objective was to determine the effects of raloxifene on fractures in a population with or at risk for CHD not selected for osteoporosis or low BMD. The data were analyzed by the sponsor according to a prespecified analysis plan. The executive committee and writing group had unrestricted request‐based access to data, which were retained by the sponsor. Data included here are those available as of February 2, 2006. All authors can vouch for the comprehensiveness and accuracy of the data and participated in interpretation of the data and drafting of the manuscript.
The protocol was approved by the institutional review board at each clinical center. All women gave written informed consent for participation in accordance with the principles of the Declaration of Helsinki.
Participants
Between 1998 and 2000, 10,101 postmenopausal women were enrolled in RUTH at 177 sites in 26 countries. Eligible women were ≥55 yr of age, ≥1 yr postmenopausal, and had established CHD or were at high risk for CHD.(4) Exclusion criteria have been described in detail elsewhere.(3‐5)
Treatment and study procedures
Eligible women were randomly assigned to raloxifene 60 mg/d orally (Evista; Eli Lilly and Company) (n = 5044) or to identical‐appearing placebo (n = 5057). Participants, investigators, laboratory and clinical center staff, and the sponsor were blinded to participants' treatment assignment. At each semiannual visit or telephone contact, adherence to study medication, adverse events, and outcomes were ascertained. Investigators were unblinded to treatment assignment only for reasons of participants' safety. The protocol for permanent or temporary discontinuation of study drug has been reported elsewhere.(5)
Assessment of risk factors for osteoporosis
Participants completed a questionnaire, were interviewed, and underwent a clinical examination at baseline. Information on participation in vigorous physical activity, alcohol intake, smoking status, family history of hip fracture, previous use of hormone therapy, and weight loss of 5 kg or more in past year was assessed by questionnaire. Women who smoked 10 or more cigarettes per day for 6 mo before enrollment were classified as current smokers. A medical history was obtained including diagnoses of prior fracture since 50 yr of age, rheumatoid arthritis, and diabetes mellitus. Diabetes mellitus for the purpose of this analysis was defined by self‐report and use of oral hypoglycemic medication or insulin or fasting baseline serum glucose level >140 mg/dl. Information on concomitant medication use including thyroid hormone, statins, thiazolidinediones, and other bone‐active agents (estrogen and/or progestin containing medications, tamoxifen, anabolic steroids, androgens, active vitamin D analogs, tibolone, bisphosphonates, calcitonin, sodium fluoride, and/or PTH) was assessed at baseline and biannual visits or telephone contacts. Weight, height, and resting pulse rate were measured at the baseline examination. Weight and height were used to calculate body mass index (BMI; kg/m2). BMD measurements were not performed.
Fracture outcomes
Fractures, a secondary endpoint of the trial, included any nonvertebral fractures (excluding fractures of the skull, hand/finger, or foot/toe) and clinical vertebral fractures. Fractures were initially reported by participants at semiannual contacts. If a fracture was reported, it was confirmed by an X‐ray report or medical record. Employees of the sponsor who were unaware of participants' treatment assignment adjudicated fracture outcomes. Nonvertebral fractures were further categorized as hip/femur and wrist fractures. Clinical vertebral fractures were defined as those that came to medical attention, were reported to the clinical centers by participants, and were confirmed with X‐ray reports or medical records.
Statistical analyses
One‐way ANOVA for continuous variables and χ2 tests for categorical variables were used to compare baseline characteristics of participants according to treatment assignment. All primary analyses used time‐to‐event methods and were based on the intention‐to‐treat principle. Kaplan‐Meier curves were generated for each specific fracture endpoint (any nonvertebral fracture, hip/femur fracture, wrist fracture, clinical vertebral fracture) for each treatment group. The log‐rank test was performed to determine whether there were differences in the curves between raloxifene and placebo groups. Cox proportional hazards regression models were used to estimate hazard ratios (HRs) and 95% CIs for raloxifene versus placebo for each fracture outcome. Secondary analyses were performed excluding women who reported use other bone‐active agents at any time during the trial, excluding women who reported use of thiazolidinediones at any time during the trial, and limiting the cohort to women who were at least 70% adherent to the study treatment on the basis of pill count.
To test whether the effect of raloxifene was consistent across categories of fracture risk, we compared the effect of raloxifene on the incidence of nonvertebral fractures and clinical vertebral fractures in risk groups defined at baseline by age (<70 versus ≥70 yr), smoking status (current smoker versus nonsmoker), physical activity level (participation in vigorous activity such as brisk walking, jogging, or swimming for ≥20 min at least once weekly versus less frequently), self‐reported prior history of fracture since age 50, family history of hip fracture (hip fracture in a first‐degree relative), diabetes mellitus, previous use of hormone therapy, thyroid hormone use, statin use, BMI (<25 versus ≥25 kg/m2), and specific fracture specific summary risk score. Summary fracture risk scores for the prediction of nonvertebral fractures and prediction of clinical vertebral fractures were developed in the placebo group using logistic regression models based on previously published methods.(6,7) Variables evaluated for inclusion in the prediction models included age, smoking status, physical activity level, alcohol intake, prior history of fracture, family history of hip fracture, rheumatoid arthritis, diabetes mellitus, previous use of hormone therapy, thyroid hormone use, statin use, weight loss of ≥5 kg in past year, resting pulse rate, and BMI. The main effect of each risk factor noted above was fit into an initial model. A stepwise selection method was used to select risk factors based on significance level of 0.05. Age, prior fracture, and family history of hip fracture were selected and included in the final nonvertebral fracture model. Age and prior use of hormone therapy were included in the final clinical vertebral fracture risk model. A summary fracture risk score for prediction of risk of nonvertebral fracture and for prediction of risk of clinical vertebral fracture for each participant was calculated using the final models. The area under the receiver operating characteristic curve was 0.60 (95% CI, 0.57‐0.62) for nonvertebral fracture and 0.68 (95% CI, 0.63‐0.73) for clinical vertebral fracture. Participants were stratified into three subgroups based on specific summary fracture risk score tertile.
To determine whether the effect of raloxifene treatment on risk of nonvertebral fractures and clinical vertebral fractures varied by fracture risk status, tests of interaction between risk factor and treatment assignment were performed. In this risk factor analysis, we examined 24 subgroups, and on average, 1 of them would be expected to be statistically significant by chance alone at the p = 0.05 level.
RESULTS
The RUTH trial randomized a total of 10,101 women: 5044 to the raloxifene group and 5057 to the placebo group. The mean age of the participants was 68 yr, and 84% were white. Approximately 39% were ≥70 yr of age. Baseline characteristics including risk factors for fracture were equally distributed between treatment groups at baseline (Table 1). Whereas only 6% of women reported a personal history of fracture, 9% reported a family history of hip fracture.
Nonvertebral fracture
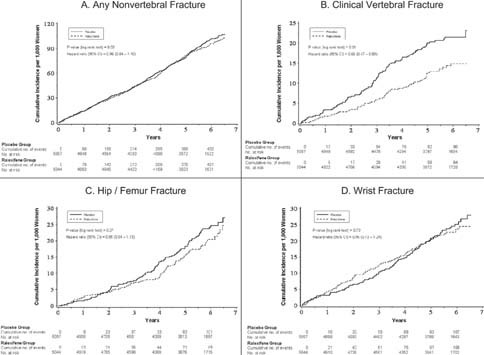
During a median follow‐up of 5.6 yr (range, 0.01‐7.06 yr), 428 women (8.5%) in the raloxifene group and 438 (8.7%) in the placebo group experienced at least one nonvertebral fracture. Fracture incidence rates per 1000 person‐years in the raloxifene and placebo groups, respectively, were 16.7 and 17.3 for nonvertebral fracture (HR, 0.96; 95% CI, 0.84‐1.10); 3.3 and 3.9 for hip/femur fracture (HR, 0.85; 95% CI, 0.64‐1.13); and 4.0 and 4.2 for wrist fracture (HR, 0.95; 95% CI, 0.73‐1.24). The cumulative incidence curves by treatment group for any nonvertebral fracture and specifically hip/femur and wrist fractures were similar (Figs. 1A, 1C, and 1D). When participants in the placebo group were stratified by tertile of the nonvertebral fracture summary risk score, the incidence rates per 1000 person‐years were 10.5 in tertile 1 (lowest), 16.9 in tertile 2 (middle), and 24.2 in tertile 3 (highest).
Cumulative incidence of fracture. (A) Any nonvertebral fracture. (B) Clinical vertebral fracture. (C) Hip/femur fracture. (D) Wrist fracture.
The absence of an effect of raloxifene treatment on nonvertebral fracture was consistent across subgroups characterized by the presence or absence of risk factors at baseline including those defined by age, smoking status, physical activity level, history of prior fracture, family history of hip fracture, diabetes mellitus, previous use of hormone therapy, thyroid hormone use, statin use, weight loss, BMI, and summary nonvertebral fracture risk score (Table 2). There was no evidence of interactions between treatment and subgroup for the prediction of risk of nonvertebral fracture (p ≥ 0.19).
Incidence of and Hazard Ratios for Any Nonvertebral Fracture by Treatment Group and Risk Subgroup
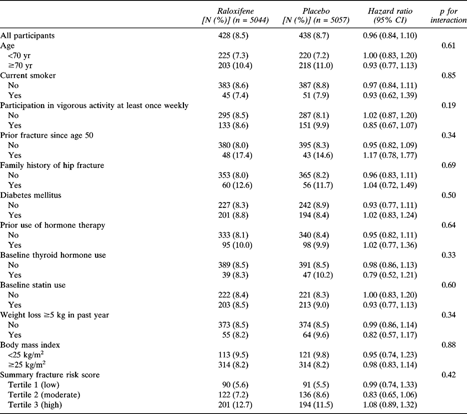
Incidence of and Hazard Ratios for Any Nonvertebral Fracture by Treatment Group and Risk Subgroup

Clinical vertebral fracture
A total of 64 women (1.3%) in the raloxifene group and 97 (1.9%) in the placebo group had a clinical vertebral fracture. The fracture incidence rate per 1000 person‐years was 2.4 in the raloxifene group and 3.7 in the placebo group; women treated with raloxifene had a 35% reduction in the risk of clinical vertebral fractures (HR, 0.65; 95% CI, 0.47‐0.89). The difference in the cumulative incidence of clinical vertebral fractures between women assigned to raloxifene and those assigned to placebo increased over time (Fig. 1B). When participants in the placebo group were stratified by tertile of the clinical vertebral fracture summary risk score, the fracture incidence rates per 1000 person‐years were 1.5 in tertile 1 (lowest), 2.7 in tertile 2 (middle), and 7.0 in tertile 3 (highest).
The effectiveness of raloxifene in reducing the risk of clinical vertebral fractures seemed to be similar in women stratified at baseline by smoking status, prior fracture since age 50, family history of hip fracture, diabetes mellitus, prior use of hormone therapy, thyroid hormone use, statin use, BMI, and summary clinical vertebral fracture risk score (p ≥ 0.37 for interaction between treatment and subgroup; Table 3). Although raloxifene seemed to be beneficial in reducing the risk of clinical vertebral fractures within subgroups defined at baseline by age, physical activity level, and weight loss in the past year, the data suggested that the relative benefit of treatment might be slightly greater in older women (p = 0.17 for interaction), those who participated in vigorous exercise (p = 0.11 for interaction), and those with weight loss (p = 0.10 for interaction).
Incidence of and Hazard Ratios for Clinical Vertebral Fracture by Treatment Group and Risk Subgroup
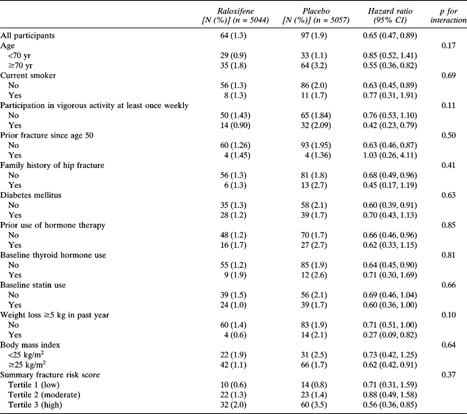
Incidence of and Hazard Ratios for Clinical Vertebral Fracture by Treatment Group and Risk Subgroup

Effects of other bone‐active agents and adherence to study medication
A total of 1455 women (14%) reported use of other bone‐active agents during the trial, including 868 women (9%) who reported bisphosphonate use. Use of other bone‐active agents was slightly more common in the placebo group compared with the raloxifene group (15% versus 14%, p = 0.02). However, the effect of raloxifene on nonvertebral and clinical vertebral fracture risk was similar when women who reported use of other bone‐active agents were excluded from the analyses (data not shown). Similarly, 402 (4%) of women reported use of a thiazolidinediones during the trial and excluding these women from our analyses did not alter the effect of raloxifene on risk of nonvertebral and clinical vertebral fracture. In addition, limiting the analyses to the cohort to the 7050 women who were at least 70% adherent to the study treatment on the basis of pill count did not alter the results (data not shown).
DISCUSSION
Treatment with raloxifene for a median of 5.6 yr in older women not selected on the basis of osteoporosis reduced the risk of clinical vertebral fractures, but not nonvertebral fractures, regardless of presence or absence of fracture risk factors.
These findings are consistent with those of the MORE trial,(2,8) conducted in 7705 postmenopausal women (mean age, 67 yr) with osteoporosis as defined by low BMD or prevalent radiographic vertebral fractures, which showed that treatment with raloxifene for 3 or 4 yr reduced the risk of new radiographic and clinical vertebral fractures but not nonvertebral fractures. Thus, the absence of an effect of raloxifene treatment in reducing risk of nonvertebral fracture in postmenopausal women not selected on the basis of increased fracture risk in our study is not unexpected. The 35% reduction in risk of clinical vertebral fractures in women unselected for osteoporosis treated with 60 mg/d of raloxifene in the RUTH trial is similar to the 35% reduction in radiographic vertebral fractures(2,8) and 41% reduction in clinical vertebral fractures(9) in women with osteoporosis treated with 60 mg/d of raloxifene for 3 yr in the MORE trial.
It is uncertain why raloxifene therapy in postmenopausal women reduces the risk of vertebral fractures without a commensurate reduction in risk of nonvertebral fractures. Evidence supporting a benefit of raloxifene in reducing risk of nonvertebral fracture in postmenopausal women is limited to a post hoc analysis(10) of the MORE trial that examined the effect of raloxifene in the 614 women with severe prevalent vertebral fractures at baseline. It has been suggested(11) that the modest increase in BMD (2‐3% after 3 yr) and normalization of the high level of bone turnover with raloxifene treatment is sufficient to prevent osteoclasts from perforating trabecular plates, reducing fractures at sites of predominately cancellous bone, such as the vertebrae; but that this modest gain in BMD and reduction in bone turnover is not be enough to reduce fractures at more cortical skeletal sites, such as the hip.(12) In RUTH, 15% of participants reported using other bone‐active agents during the trial, including more potent antiresorptive agents such as bisphosphonates, and use of these agents was slightly more common among women assigned to placebo. However, exclusion of women taking bone‐active agents from the analysis did not alter our results.
Among postmenopausal women without osteoporosis, evidence for the benefit of pharmacologic therapy in preventing fractures, especially nonvertebral fractures, is limited. A large placebo‐controlled trial of tamoxifen,(13) another SERM, did not find a significant reduction in osteoporotic fractures (including hip, clinical vertebral, or wrist fractures) at 5 yr among women assigned to tamoxifen. The large Women Health Initiative (WHI) trials of estrogen plus progestin(7) and estrogen alone(14) in healthy postmenopausal women (mean age, 63 and 64 yr, respectively) showed that hormone therapy reduced the risk of total osteoporotic fractures, including hip and clinical vertebral fractures. However, in the Heart and Estrogen/Progestin Replacement Study (HERS), a trial of postmenopausal women (mean age, 67 yr) with coronary heart disease,(15,16) there was no evidence that treatment with estrogen plus progestin reduced risk of fractures, including clinical vertebral and nonvertebral fractures. It is not known why there is a discrepancy in findings between WHI and HERS, but it has been at least partially attributed to differences in study populations and sample sizes in the trials.(7)
With respect to effects of the more potent antiresorptive agents (such as the bisphosphonates) in preventing fractures among women without osteoporosis, a trial of alendronate in postmenopausal women with low BMD (BMD T‐score, −1.6 or below) without prevalent vertebral fractures(17) found that alendronate reduced the risk of clinical (nonvertebral and clinical vertebral) fractures among women with osteoporosis (BMD T‐score, −2.5 or below), but not among women with higher BMD. A secondary analysis of women without osteoporosis (BMD T‐score > −2.5) enrolled in this trial(18) reported no effect of alendronate treatment on risk of nonvertebral fracture, even among the high risk subgroup of women with a prior history of fracture. A trial of risedronate therapy(19) that enrolled women ≥80 yr of age at high risk of hip fracture based on the presence of multiple clinical risk factors, but not selected on the basis of low BMD, showed no effect of treatment in reducing risk of hip or other nonvertebral fractures. A single site recent trial of clodronate therapy in unselected community‐dwelling women ≥75 yr of age showed a 20% reduction in risk of all clinical fractures but no evidence of an effect on hip fractures.(20)
We found that the benefit of raloxifene therapy in reducing risk of clinical vertebral fractures and its lack of effect on risk of nonvertebral fractures was consistent within categories of fracture risk defined at baseline by age, smoking status, physical activity level, prior history of fracture, family history of hip fracture, diabetes mellitus, previous use of hormone therapy, thyroid hormone use, statin use, weight loss, and BMI. In addition, there was no evidence that the effect of raloxifene treatment on risk of fracture differed according to summary fracture risk score. For example, we found that raloxifene treatment reduced clinical vertebral fractures to the same degree among women at low, intermediate, and high risk of this fracture. Whereas several osteoporosis risk assessment tools(21‐23) have been developed to identify postmenopausal women at high risk of osteoporosis who should have BMD testing, more recent efforts led by the World Health Organization(24) have focused on the identification of validated clinical risk factors that may be used with and without BMD to identify those at high risk of fracture. Although we evaluated several potential risk factors and the increase in incidence rates from the lowest to highest risk tertile in our summary fracture risk scores increased 2‐fold for nonvertebral fracture and >4‐fold for clinical vertebral fracture, few factors were selected for inclusion in our summary scores beyond age alone. The area under the curve (AUC) statistics from receiver operating characteristic curves indicated that the scores were limited in their ability to discriminate nonvertebral and clinical vertebral fracture. However, the predictive validity of these scores was similar to that reported for other tools in predicting osteoporotic fractures based on the use of clinical risk factors alone.(25)
Evidence exists to support interrelationships between osteoporosis and vascular calcification or CHD in older women, but it is uncertain whether these conditions are independent age‐related processes or linked by similar biological pathways. Some,(26) but not all,(27,28) cross‐sectional studies in middle‐aged and older women have reported an association between low BMD and greater coronary artery calcification. Similarly, some,(29,30) but not all,(31) prospective studies have reported an association between osteoporosis in women as defined by low BMD and/or the presence of prevalent vertebral deformities and increased risk of incident cardiovascular disease. To our knowledge, no prior prospective study has examined whether prevalent CHD in older women is independently associated with an increased risk of fractures. However, among postmenopausal women with heart disease enrolled in the HERS trial, those with fractures had a subsequent lower, not higher, risk of incident coronary events.(32)
Both obesity and diabetes mellitus were common in our study population of older women selected on the basis of elevated risk for coronary events, and the presence of these conditions might substantially influence risk for fracture. Whereas low body weight confers an elevated risk of fracture, in particular hip fracture,(33) it is uncertain whether obese women are protected from fracture. For example, prospective studies have suggested that overweight and obese women compared with women of normal body weight have a small reduction(33) or no reduction(34) in the risk of hip fracture. With regard to diabetes, data from most prospective cohort studies suggest that adults with type 2 diabetes mellitus have a higher risk of fracture compared with nondiabetic adults(35‐37) despite their higher BMD(38) and body weight that are associated with diabetes. However, we found no evidence that the effect of raloxifene treatment on risk of fracture differed according to body weight category or diabetes status. In addition, although use of thiazolidinediones has been linked to higher rates of bone loss(39,40) and an increased risk of fracture,(41) only 4% of women reported use of thiazolidinediones during the trial, and excluding them from the analyses did not alter our results.
The RUTH trial has a number of strengths including its randomized design, global enrollment, and fracture confirmation using X‐ray reports and/or medical records. However, there are several limitations. The study population was comprised of predominantly white women selected on the basis of elevated risk for coronary events, and our findings may not be generalizable to other populations. The risk subgroup analysis was prespecified, but our results are subject to the limitations of subgroup analyses.(42) Although we found no evidence to suggest that treatment with raloxifene was beneficial in some subgroups and harmful in others, our power is insufficient to conclude that there is no small difference in magnitude of effect between subgroups. BMD and the presence of prevalent vertebral fractures are strong risk factors for subsequent fractures but were not assessed in the RUTH trial. Our analysis did not include incident radiographically identified vertebral deformities and previous studies suggest that only one fourth(43) to one third(44) of incident radiographic vertebral deformities are clinically recognized. However, clinical vertebral fractures are strongly associated with adverse health outcomes including lower health‐related quality of life,(45) fracture‐related disability,(46) an increased risk of subsequent clinical fractures,(47) and increased mortality.(48,49) Nonvertebral fractures are a heterogenous group, but our study was not powered to examine the effect of raloxifene treatment on specific types of nonvertebral fractures.
In conclusion, treatment with raloxifene for 5 yr in older women not selected on the basis of osteoporosis or increased fracture risk effectively reduces the risk of clinical vertebral fractures but not nonvertebral fractures. These effects of raloxifene therapy were consistently observed, irrespective of the presence or absence of fracture risk factors.
Acknowledgements
We are indebted to the 10,101 women, the investigators, and the staff for their dedication and commitment to the RUTH trial; to Kyle Moen for preparation of the figures and assistance with the manuscript; and to Gail P Dalsky for input into the scientific content of the manuscript. This study was funded by Eli Lilly and Company, Indianapolis, IN, USA.
Reference
Author notes
Dr Ensrud received research grant support through contracts with the University of Minnesota from Eli Lilly and Company, Pfizer, Merck, Roche, Berlex, and Bionovo. Dr Stock is a full‐time employee of Eli Lilly and Company. Dr Barrett‐Connor received research grant support through contracts with the University of California‐San Diego from Amgen, Merck, Organon, Envision Pharma, and Eli Lilly and Company. Dr Grady received research grant support through contracts with the University of California‐San Francisco from Berlex, Bionovo, Eli Lilly and Company, and Pfizer and research and consulting fees for chairing a data and safety monitoring board at Organon. Dr Mosca received consulting fees from Pfeizer, Organon, Merck, Sheray Plough, Novartis, Wyeth, Amgen, and Eli Lilly and Company. Dr Khaw received consultant fees for endpoint adjudication from Eli Lilly and Company. Drs Zhao and Agnusdei are full‐time employees of Eli Lilly and Company. Dr Cauley received research grant support through contracts with the University of Pittsburgh from Merck & Company, Pfizer Pharmaceuticals, Novartis Pharmaceuticals, and Eli Lilly and Company; honorarium from Merck & Company, Novartis, and Eli Lilly and Company, and speaker's bureau for Merck and Company.