Abstract
Background
The objective of this study was to analyze factors leading to explantation of totally implanted access ports (TIAPs) and to assess its occurrence and clinical relevance.
Methods
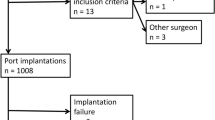
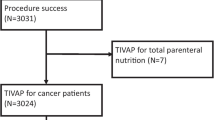
Of 438 patient consecutive patients with a port explantation, 385 were eligible for this retrospective cohort study. Reasons for explantation as well as demographic, clinical, and surgical characteristics were analyzed by univariate and multivariate models.
Results
The diagnoses leading to TIAP implantation were hematological malignancies in 142 patients (36.8%), breast cancer in 103 patients (26.8%), gastrointestinal cancer in 76 patients (19.8%), nonmalignant diseases in 46 patients (11.9%), and other malignant diseases in 18 patients (4.7%). The reasons for TIAP explantation were infection in 178 patients (46.2%), end of treatment in 129 patients (33.5%), thrombosis in 44 patients (11.4%), TIAP dysfunction in 22 patients (5.7%), and other reasons in 12 patients (3.2%). At the time of TIAP explantation, 115 patients (29.9%) were receiving chemotherapy, and 49 patients (12.7%) were considered immunocompromised. In case of TIAP explantation due to infection, the median length of TIAP in situ time was 303.3 days, whereas the cumulative 10-day and 30-day explantation rates were 2.8% and 10.6%, respectively. By multivariate models, TIAP explantation due to infection is statistically significantly decreased in patients with breast cancer (P < .01) but significantly increased in patients with recurrent TIAP implantation and with ongoing chemotherapy (P < .01).
Conclusions
TIAP explantations are caused primarily by late-term complications, mainly infections. The subsequent interruption of ongoing treatment makes further efforts necessary to reduce such complications.

Similar content being viewed by others
References
Broviac JW, Cole JJ, Scribner BH. A silicone rubber atrial catheter for prolonged parenteral alimentation. Surg Gynecol Obstet 1973;136:602–6
Seiler CM, Frohlich BE, Dorsam UJ, et al. Surgical technique for totally implantable access ports (TIAP) needs improvement: a multivariate analysis of 400 patients. J Surg Oncol 2006;93:24–9
Niederhuber JE, Ensminger W, Gyves JW, et al. Totally implanted venous and arterial access system to replace external catheters in cancer treatment. Surgery 1982;92:706–12
Adler A, Yaniv I, Steinberg R, et al. Infectious complications of implantable ports and Hickman catheters in paediatric haematology-oncology patients. J Hosp Infect 2006;62:358–65
Ng F, Mastoroudes H, Paul E, et al. A comparison of Hickman Line– and Port-a-Cath–associated complications in patients with solid tumours undergoing chemotherapy. Clin Oncol (R Coll Radiol) 2007; 19:551–6
Knaebel HP, Marten A, Schmidt J, et al. Phase III trial of postoperative cisplatin, interferon alpha-2b, and 5-FU combined with external radiation treatment versus 5-FU alone for patients with resected pancreatic adenocarcinoma—CapRI: study protocol. BMC Cancer 2005;5:37
Wu AW, Xu GW, Wang HY, et al. Neoadjuvant chemotherapy versus none for resectable gastric cancer. Cochrane Database Syst Rev 2007;CD005047
Biffi R, de Braud F, Orsi F, et al. Totally implantable central venous access ports for long-term chemotherapy. A prospective study analyzing complications and costs of 333 devices with a minimum follow-up of 180 days. Ann Oncol 1998;9:767–73
Biffi R, Pozzi S, Pace U, et al. Is there a real advantage in utilizing central venous ports in oncology surgery? An analysis of the cost-effectiveness ratio. Tumori 2001;87:S74–5
Kock HJ, Pietsch M, Krause U, et al. Implantable vascular access systems: experience in 1500 patients with totally implanted central venous port systems. World J Surg 1998;22:12–6
Di Carlo I, Cordio S, La Greca G, et al. Totally implantable venous access devices implanted surgically: a retrospective study on early and late complications. Arch Surg 2001;136:1050–3
Shetty PC, Mody MK, Kastan DJ, et al. Outcome of 350 implanted chest ports placed by interventional radiologists. J Vasc Interv Radiol 1997;8:991–5
Zieschang M, Erben B, Hoffler D, et al. The Demers atrial catheter: experience with a single-lumen silicone catheter as short- and long-term access for hemodialysis. Clin Nephrol 1995;44:113–7
Hickman RO, Buckner CD, Clift RA, et al. A modified right atrial catheter for access to the venous system in marrow transplant recipients. Surg Gynecol Obstet 1979;148:871–5
Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992;101:1644–55
International Agranulocytosis and Aplastic Anemia Study. Incidence of aplastic anemia: the relevance of diagnostic criteria. By the International Agranulocytosis and Aplastic Anemia Study. Blood 1987;70:1718–21
Pagani O, Gelber S, Price K, et al. Toremifene and tamoxifen are equally effective for early-stage breast cancer: first results of International Breast Cancer Study Group Trials 12–93 and 14–93. Ann Oncol 2004;15:1749–59
Hotta T, Takifuji K, Arii K, et al. Toxicity during L-LV/5FU adjuvant chemotherapy as a modified RPMI regimen for patients with colorectal cancer. Oncol Rep 2005;14:433–9
Gastmeier P, Geffers C. Prevention of catheter-related bloodstream infections: analysis of studies published between 2002 and 2005. J Hosp Infect 2006;64:326–35
Carmeli Y, Castro J, Eliopoulos GM, et al. Clinical isolation and resistance patterns of and superinfection with 10 nosocomial pathogens after treatment with ceftriaxone versus ampicillin-sulbactam. Antimicrob Agents Chemother 2001;45:275–9
Aliyu SH, Enoch DA, Abubakar II, et al. Candidaemia in a large teaching hospital: a clinical audit. QJM 2006;99:655–63
Ozkocaman V, Ozcelik T, Ali R, et al. Bacillus spp among hospitalized patients with haematological malignancies: clinical features, epidemics and outcomes. J Hosp Infect 2006;64:169–76
Prevention of vascular catheter associated infections. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz 2002:907–24
O’Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter-related infections. Centers for Disease Control and Prevention. MMWR Recomm Rep 2002;51:1–29
Veenstra DL, Saint S, Saha S, et al. Efficacy of antiseptic-impregnated central venous catheters in preventing catheter-related bloodstream infection: a meta-analysis. JAMA 1999;281:261–7
Darouiche RO, Raad II, Heard SO, et al. A comparison of two antimicrobial-impregnated central venous catheters. Catheter Study Group. N Engl J Med 1999;340:1–8
Gastmeier P, Zuschneid I, Geffers C. Antimicrobially impregnated catheters: An overview of randomized controlled trials. J Vasc Access 2003;4:102–10
Chiou PF, Chang CC, Wen YK, et al. Antibiotic lock technique reduces the incidence of temporary catheter-related infections. Clin Nephrol 2006;65:419–22
Scalamogna A, Castelnovo C, De Vecchi A, Ponticelli C. Exit-site and tunnel infections in continuous ambulatory peritoneal dialysis patients. Am J Kidney Dis 1991;18:674–7
ACKNOWLEDGMENT
This study was sponsored in part by Vygon GmbH & Co. KG, Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
Lars Fischer and Phillip Knebel contributed equally to this study.
Rights and permissions
About this article
Cite this article
Fischer, L., Knebel, P., Schröder, S. et al. Reasons for Explantation of Totally Implantable Access Ports: A Multivariate Analysis of 385 Consecutive Patients. Ann Surg Oncol 15, 1124–1129 (2008). https://doi.org/10.1245/s10434-007-9783-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-007-9783-z