-
PDF
- Split View
-
Views
-
Cite
Cite
Sabine Holst, Silke Laakmann, Morphological and molecular discrimination of two closely related jellyfish species, Cyanea capillata and C. lamarckii (Cnidaria, Scyphozoa), from the northeast Atlantic, Journal of Plankton Research, Volume 36, Issue 1, January/February 2014, Pages 48–63, https://doi.org/10.1093/plankt/fbt093
Close - Share Icon Share
Abstract
Detecting fluctuations in the species composition of bloom-forming jellyfish requires the ability to correctly identify each species in each developmental stage. We verified diagnostic morphological and molecular genetic characters to discriminate Cyanea lamarckii and Cyanea capillata from northern European waters. Intrusions in the subumbrellar muscle folds were present in all C. capillata >80 mm r-diameter (between opposite rhopalia tips), but absent in C. lamarckii. Clearly visible wart-like papillae on the central exumbrella were present in all C. lamarckii >10–80 mm r-diameter, but absent in C. capillata. Both morphological features were retained in formaldehyde-seawater (4%) preserved medusae which had shrunk by 12.8% (±2.7%) after 1 year of preservation. Our molecular genetic analyses demonstrated that fragments of mitochondrial cytochrome c oxidase subunit I (COI) and nuclear 18S rDNA clearly distinguished C. lamarckii from C. capillata, with intra- and inter-specific pairwise genetic distances of 0.0–1.5% and 15.5–17.0% (COI) and 0.0 and 0.2% (18S rDNA), respectively. The study revealed various bell colours in both species underlining that the identification based on the bell colours can result in misidentification. Our integrated taxonomic approach can help to correctly identify jellyfish species, which is fundamentally important for understanding the causes of jellyfish fluctuations and the development of jellyfish blooms.
INTRODUCTION
Scientific interest in jellyfish abundance and distribution has increased in recent decades, when conflicts between mass occurrences and socioeconomic interests, such as fisheries, industries and tourism became obvious (Purcell et al., 2007; Richardson et al., 2009; Purcell, 2012; Gibbons and Richardson, 2013). Causes of jellyfish blooms and the role of human-mediated environmental change in alternations of jellyfish biomass are still unclear (Condon et al., 2013; Lee et al., 2013). Monitoring of jellyfish in northern European waters has been conducted by net captures (e.g. Lynam et al., 2011), Continuous Plankton Recorder surveys (Gibbons and Richardson, 2009), observations by airplane (Houghton et al., 2006) and surface jellyfish counts from ships and shoreline surveys (Doyle et al., 2007). The correct species identification can be problematic with these monitoring methods, but it is fundamental for the detection of the drivers of specific jellyfish fluctuations.
In the northeast Atlantic, morphological differentiation to the genus level is relatively easy in adult medusae because identification features are quite obvious, whereas it is more difficult to distinguish the young medusa stages (Russell, 1970; Holst, 2012a). Two species of the genus Cyanea, C. capillata (Linnaeus, 1758) and C. lamarckii (Péron and Lesueur, 1809), occur in northern European waters and can reach high abundances (Hay et al., 1990; Barz and Hirche, 2007). Owing to their very similar morphology, identification often has been restricted to genus (Cyanea spp., e.g. Möller, 1980; Lynam et al., 2011), or may have led to errors (Houghton et al., 2006). Their distribution overlaps west of Denmark, in the German Bight, and the southern North Sea, where C. lamarckii medusae tend to appear earlier and more abundantly than C. capillata (Hagmeier, 1930; Künne, 1952; Russell, 1970; Hay et al., 1990; Barz and Hirche, 2007). C. capillata regularly occurs in the western part of the Baltic Sea at low salinities up to the Gulf of Finland (Haahtela and Lassig, 1967; Holst and Jarms, 2010). In contrast, the distribution of C. lamarckii in the Baltic is mainly limited to the west coast of Sweden (Kramp, 1961; Gröndahl, 1988) and its appearance in more southern parts is exceptional (Russell, 1970; Rasmussen, 1973).
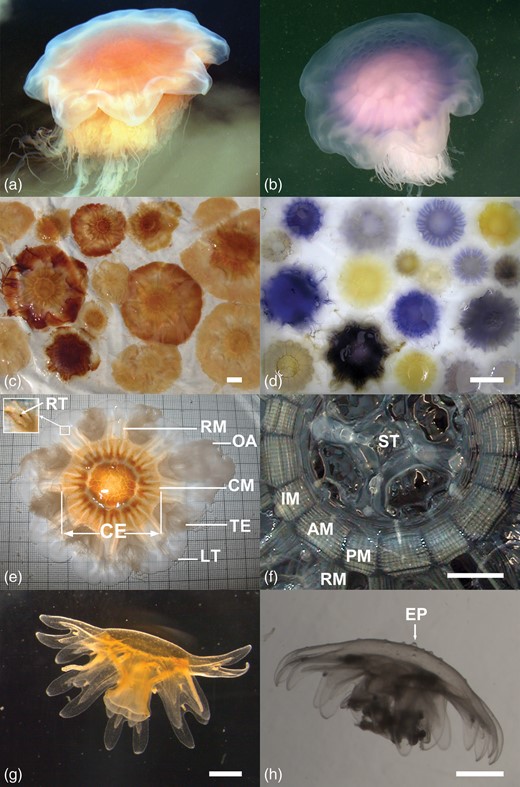
C. capillata from the North Sea is the type species of the genus Cyanea (Péron and Lesueur, 1809). C. lamarckii was regarded as a valid species by several authors (e.g. Agassiz, 1862; Haeckel, 1880; Hartlaub, 1894; Delap, 1905; Vanhöffen, 1906; Kramp, 1939), but it was suggested to be a juvenile stage by others (e.g. Stiasny, 1930), or a variety of C. capillata (e.g. Mayer, 1910). Stiasny and Maaden (Stiasny and Maaden, 1943) did not find any morphological differences between C. lamarckii and C. capillata and combined them as one species (C. capillata), although they remarked that the medusae of both forms may vary in colour, size and seasonal occurrence. C. lamarckii is called the “blue jellyfish” in different languages (UK: blue hair jelly, G: blaue Nesselqualle, NL: blauwe haarquall, DEN: blå brandmand, E: Medusa orticante azul, F: Cyanée bleue). The blue bell colour has often been described as a typical character for C. lamarckii medusae and was used to distinguish them from C. capillata medusae (e.g. Vanhöffen, 1906; Kramp, 1961; Fraser, 1972); however, bell colours can vary enormously, from pale yellow to blue or violet in C. lamarckii and from yellowish brown, orange brown or reddish in C. capillata (Thiel, 1962; Russell, 1970) (Fig. 1a–d). C. capillata usually is <500 mm bell diameter in British waters, but can exceptionally grow to >900 mm. In contrast, C. lamarckii is smaller, usually being <150 mm and 300 mm maximum (Russell, 1970). However, medusae of both species similar in size occur together temporally in the same locality (Hay et al., 1990; Barz and Hirche, 2007). In conclusion, size is not a distinguishing character and identification based on colour can cause errors in yellowish-coloured individuals.
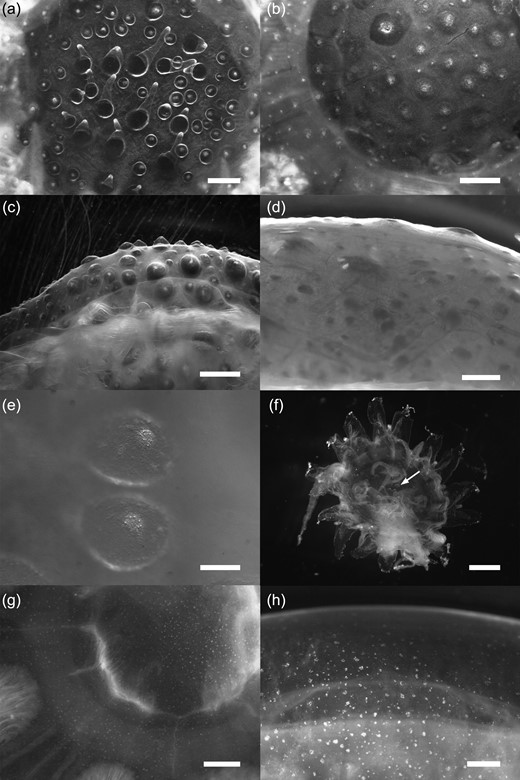
Morphology and colour variations in Cyanea capillata and Cyanea lamarckii. (a) C. capillata, free swimming medusa, (b) C. lamarckii, free swimming medusa with papillose exumbrella, (c) C. capillata, colour variations in medusae from Kiel Bight, (d) C. lamarckii, colour variations in medusae from Helgoland, (e) C. capillata morphology, view at the exumbrella, (f) C. capillata, view at the subumbrellar musculature (oral arms and gonads removed), (g) C. capillata, young medusa 6 weeks after release from the polyp, smooth exumbrella with nematocyst clusters visible as white dots, (h) C. lamarckii, young medusa 4 weeks after release from the polyp with exumbrella papillae. AM, adradial muscle field; CE, central exumbrella; CM, circular musculature; EP, exumbrella papilla; IM, interradial muscle field; LT, marginal lappet tip; OA, oral arm; PM, perradial muscle field; RM, radial muscle; RT, rhopalia tip; ST, stomach; TE, tentacles. Scale bars: (c, d, f) = 50 mm; (g, h) = 1 mm.
Thiel (Thiel, 1962) was the first to conduct detailed morphological comparisons of Cyanea sp. medusae of different sizes from North and Baltic seas. He found significantly more tentacles (on average twice the number) relative to bell size in C. capillata compared with C. lamarckii, thereby establishing the first certain character to distinguish the two species (Russell, 1970). Counting tentacles, however, is difficult, extremely time consuming and much too cumbersome for a quick and certain species identification. Russel (Russell, 1970) found other differences between the two Cyanea species; in the muscle folds on the umbrella underside (subumbrella), C. capillata has fewer folds in the circular muscle between the radial septa than C. lamarckii; also, pit-like intrusions from the gastrovascular sinus into the circular and radial muscle folds are present in C. capillata but absent or few in C. lamarckii. The number of muscle folds is difficult to count (Russell, 1970), but the intrusions in the muscle folds are visible by eye in adult medusae and, therefore, applicable for quick species determination (Holst, 2012a). Unfortunately, as noted by Russell (Russell, 1970), this character does not develop until the C. capillata medusae are ∼30 mm in diameter and is an uncertain feature for the identification of smaller specimens.
Another morphological difference between the two species mentioned by several authors is the structure of the medusa bell surface (exumbrella), which was described as more papillose in C. lamarckii than in C. capillata. This difference was never defined as a distinguishing feature of the two species, however, because it was assumed to be present in small specimens only (Hagmeier, 1930; Thiel, 1962). In his morphological description of C. lamarckii, Russell (Russell, 1970) stated, “The surface of the exumbrella is slightly papillose, especially in its central region where the scattered nematocyst clusters are particularly prominent. These can easily be seen by removing the medusa from the water and viewing the exumbrella at an angle to the incident light”. For C. capillata he stated, “The exumbrellar surface is faintly papillose in the peripheral region due to clusters of the nematocysts, but the central part of the disk is smooth”.
The aim of the present study is to provide a useful method for certain and practicable morphological discrimination between C. capillata and C. lamarckii medusae in different developmental stages. For both species, >100 specimens of <10 to >100 bell diameter were investigated morphologically to verify the applicability of two size-dependent identification features: (i) the presence of intrusions in the muscle folds on the subumbrella, and (ii) the presence of prominent papillae at the central exumbrella. Both features were examined in fresh material and were re-examined in medusae after 1- and 2-years storage in a formaldehyde seawater solution (4%). Shrinkage after 1 year preservation was calculated by measurements of the fresh and preserved medusae. In addition to the specimens collected from the North Sea (C. lamarckii) and Baltic Sea (C. capillata), ephyrae of both species were reared from North Sea polyps to observe the appearance of exumbrella papillae in these youngest medusa stages.
Our morphological analyses were supplemented by molecular analyses. The latter are increasingly applied in studies of the Scyphozoa, focusing on phylogeny (Bayha et al., 2010), species discrimination (Dawson and Jacobs, 2001; Dawson, 2005a; Bayha and Dawson, 2010; Galil et al., 2010; Ortman et al., 2010), and phylogeographic patterns (Holland et al., 2004; Stopar et al., 2010; Lee et al., 2013). These studies have shown that species discrimination and identification is very successful based on the sequence divergence of a fragment of mitochondrial cytochrome c oxidase subunit I (COI). Some of those studies highlighted putative or cryptic species in different jellyfish species (e.g. Dawson and Jacobs, 2001; Holland et al., 2004; Dawson, 2005a). For Cyanea species, the generally accepted cosmopolitan distribution of C. capillata (e.g. Kramp, 1961) was rebutted by Dawson (Dawson, 2005a), who found Cyanea species from Australian waters to be genetically different from C. capillata collected in the North Sea. In our study, we used molecular genetic differentiation on C. lamarckii sampled from the North Sea and cultured C. capillata, as well as sampled from the North Sea, Baltic Sea and Icelandic waters. For this, both fast and slowly evolving markers (i.e. fragments of mitochondrial COI and nuclear 18S rDNA) were analyzed together with published sequence data on other Cyanea species available in GenBank.
METHOD
Morphological examinations
Morphology first was investigated on fresh material, 85 C. lamarckii medusae collected in Helgoland marina (North Sea) in May and June 2010 and 228 C. capillata medusae collected in Strande marina (Baltic Sea, see Table I) in May to September 2009. Medusae were collected with a sampling bucket or a hand net and transported to the laboratory in buckets with seawater. Investigations were conducted in the laboratories of the Biologische Anstalt Helgoland, Alfred Wegener Institute (C. lamarckii) and the DZMB Hamburg, Biocenter Grindel (C. capillata). Live medusae were examined and re-examined after 1- and 2-years storage in a borax buffered 4% formaldehyde-seawater solution (hereafter referred to as “4% formaldehyde”) as follows:
To detect papillae on the central exumbrella, defined as the exumbrella region located within the subumbrellar ring musculature visible through the bell (Fig. 1e), each medusa was taken out of the liquid (seawater or 4% formaldehyde). The exumbrella was examined from the top and also from the side if no papillae were visible from the top. Four categories were defined for the visibility of exumbrella papillae: C1: Conspicuous, C2: Flat or few but clearly visible, C3: Inconspicuous and hardly visible and C4: None visible.
Two bell diameters were measured to 1-mm accuracy with the aid of a calibration foil (a millimetre scale printed on a film; Fig. 1e): “total diameter” between opposite lappet tips (Fig. 1e), hereafter referred to as “t-diameter”, and “rhopalar diameter” between opposite rhopalia tips (Fig. 1e), hereafter “r-diameter”.
To examine the subumbrella musculature, the medusa was turned onto the exumbrellar side. Four adjacent adradial muscle fields (Fig. 1f) were examined and the maximal number of intrusions in one muscle fold was determined.
A stereomicroscope was used for all examinations and measurements of specimens <20 mm r-diameter.
Cyanea lamarckii and C. capillata samples included in the molecular genetic analyses
| Sampling . | Origin . | Bell colour . | GenBank Acc. No. . | ||||
|---|---|---|---|---|---|---|---|
| Region . | Latitude . | Longitude . | Date . | COI . | 18S rDNA . | ||
| Cyanea lamarckii | |||||||
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2011 | Sampled | Colourless | JX995347 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2011 | Sampled | Colourless | JX995348 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2011 | Sampled | Colourless | JX995349 | |
| NS; German Bight | 53.7645 | 8.0629 | June 2011 | Sampled | Violet | JX995350 | |
| NS; German Bight | 53.7645 | 8.0629 | June 2011 | Sampled | Violet | JX995351 | |
| NS; German Bight | 53.7645 | 8.0629 | June 2011 | Sampled | Yellow | JX995352 | |
| NS; German Bight | 53.7645 | 8.0629 | June 2011 | Sampled | White | JX995353 | |
| NS; German Bight | 53.7645 | 8.0629 | June 2011 | Sampled | Blue | JX995354 | |
| NS; German Bight | 53.9491 | 6.7604 | June 2011 | Sampled | Yellow–white | JX995355 | |
| NS; German Bight | 54.5662 | 7.1468 | June 2011 | Sampled | Violet | JX995356 | JX995325 |
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2010 | Sampled | Blue | JX995357 | JX995326 |
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2010 | Sampled | Pale yellow | JX995360 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2010 | Sampled | Pale yellow | JX995361 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2010 | Sampled | Pale yellow | JX995362 | |
| Cyanea capillata | |||||||
| BS; Aarøsund Marina | 55.2574 | 9.7069 | June 2011 | Sampled | Pale orange | JX995330 | |
| BS; Aarøsund Marina | 55.2574 | 9.7069 | June 2011 | Sampled | Pale orange | JX995331 | |
| BS; Aarøsund Marina | 55.2574 | 9.7069 | June 2011 | Sampled | Pale orange | JX995332 | |
| BS; Aarøsund Marina | 55.2574 | 9.7069 | June 2011 | Sampled | Pale orange | JX995333 | |
| BS; Aarøsund Marina | 55.2574 | 9.7069 | June 2011 | Sampled | Pale orange | JX995334 | JX995327 |
| NS; Helgoland Roads | 54.1863 | 7.9000 | June 2011 | Culture | Orange | JX995335 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | June 2011 | Culture | Orange | JX995336 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | June 2011 | Culture | Orange | JX995337 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | June 2011 | Culture | Orange | JX995338 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | June 2011 | Culture | Orange | JX995339 | JX995328 |
| Icelandic waters | 67.6333 | −12.1667 | Sept 2011 | Sampled | Pale orange | JX995340 | |
| BS; Strande Marina | 54.4352 | 10.1701 | June 2009 | Sampled | Pale orange | JX995341 | |
| BS; Strande Marina | 54.4352 | 10.1701 | June 2009 | Sampled | Pale orange | JX995342 | |
| BS; Strande Marina | 54.4352 | 10.1701 | June 2009 | Sampled | Pale orange | JX995343 | |
| BS; Strande Marina | 54.4352 | 10.1701 | May 2009 | Sampled | Orange–brown | JX995344 | |
| BS; Strande Marina | 54.4352 | 10.1701 | May 2009 | Sampled | Pale orange | JX995345 | |
| BS; Strande Marina | 54.4352 | 10.1701 | May 2009 | Sampled | Orange–brown | JX995346 | |
| Aurelia aurita (outgroup taxa) | |||||||
| BS; Fehmarn Belt | Dec 2010 | JX995329 | |||||
| Sampling . | Origin . | Bell colour . | GenBank Acc. No. . | ||||
|---|---|---|---|---|---|---|---|
| Region . | Latitude . | Longitude . | Date . | COI . | 18S rDNA . | ||
| Cyanea lamarckii | |||||||
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2011 | Sampled | Colourless | JX995347 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2011 | Sampled | Colourless | JX995348 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2011 | Sampled | Colourless | JX995349 | |
| NS; German Bight | 53.7645 | 8.0629 | June 2011 | Sampled | Violet | JX995350 | |
| NS; German Bight | 53.7645 | 8.0629 | June 2011 | Sampled | Violet | JX995351 | |
| NS; German Bight | 53.7645 | 8.0629 | June 2011 | Sampled | Yellow | JX995352 | |
| NS; German Bight | 53.7645 | 8.0629 | June 2011 | Sampled | White | JX995353 | |
| NS; German Bight | 53.7645 | 8.0629 | June 2011 | Sampled | Blue | JX995354 | |
| NS; German Bight | 53.9491 | 6.7604 | June 2011 | Sampled | Yellow–white | JX995355 | |
| NS; German Bight | 54.5662 | 7.1468 | June 2011 | Sampled | Violet | JX995356 | JX995325 |
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2010 | Sampled | Blue | JX995357 | JX995326 |
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2010 | Sampled | Pale yellow | JX995360 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2010 | Sampled | Pale yellow | JX995361 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2010 | Sampled | Pale yellow | JX995362 | |
| Cyanea capillata | |||||||
| BS; Aarøsund Marina | 55.2574 | 9.7069 | June 2011 | Sampled | Pale orange | JX995330 | |
| BS; Aarøsund Marina | 55.2574 | 9.7069 | June 2011 | Sampled | Pale orange | JX995331 | |
| BS; Aarøsund Marina | 55.2574 | 9.7069 | June 2011 | Sampled | Pale orange | JX995332 | |
| BS; Aarøsund Marina | 55.2574 | 9.7069 | June 2011 | Sampled | Pale orange | JX995333 | |
| BS; Aarøsund Marina | 55.2574 | 9.7069 | June 2011 | Sampled | Pale orange | JX995334 | JX995327 |
| NS; Helgoland Roads | 54.1863 | 7.9000 | June 2011 | Culture | Orange | JX995335 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | June 2011 | Culture | Orange | JX995336 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | June 2011 | Culture | Orange | JX995337 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | June 2011 | Culture | Orange | JX995338 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | June 2011 | Culture | Orange | JX995339 | JX995328 |
| Icelandic waters | 67.6333 | −12.1667 | Sept 2011 | Sampled | Pale orange | JX995340 | |
| BS; Strande Marina | 54.4352 | 10.1701 | June 2009 | Sampled | Pale orange | JX995341 | |
| BS; Strande Marina | 54.4352 | 10.1701 | June 2009 | Sampled | Pale orange | JX995342 | |
| BS; Strande Marina | 54.4352 | 10.1701 | June 2009 | Sampled | Pale orange | JX995343 | |
| BS; Strande Marina | 54.4352 | 10.1701 | May 2009 | Sampled | Orange–brown | JX995344 | |
| BS; Strande Marina | 54.4352 | 10.1701 | May 2009 | Sampled | Pale orange | JX995345 | |
| BS; Strande Marina | 54.4352 | 10.1701 | May 2009 | Sampled | Orange–brown | JX995346 | |
| Aurelia aurita (outgroup taxa) | |||||||
| BS; Fehmarn Belt | Dec 2010 | JX995329 | |||||
Cyanea lamarckii and C. capillata samples included in the molecular genetic analyses
| Sampling . | Origin . | Bell colour . | GenBank Acc. No. . | ||||
|---|---|---|---|---|---|---|---|
| Region . | Latitude . | Longitude . | Date . | COI . | 18S rDNA . | ||
| Cyanea lamarckii | |||||||
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2011 | Sampled | Colourless | JX995347 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2011 | Sampled | Colourless | JX995348 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2011 | Sampled | Colourless | JX995349 | |
| NS; German Bight | 53.7645 | 8.0629 | June 2011 | Sampled | Violet | JX995350 | |
| NS; German Bight | 53.7645 | 8.0629 | June 2011 | Sampled | Violet | JX995351 | |
| NS; German Bight | 53.7645 | 8.0629 | June 2011 | Sampled | Yellow | JX995352 | |
| NS; German Bight | 53.7645 | 8.0629 | June 2011 | Sampled | White | JX995353 | |
| NS; German Bight | 53.7645 | 8.0629 | June 2011 | Sampled | Blue | JX995354 | |
| NS; German Bight | 53.9491 | 6.7604 | June 2011 | Sampled | Yellow–white | JX995355 | |
| NS; German Bight | 54.5662 | 7.1468 | June 2011 | Sampled | Violet | JX995356 | JX995325 |
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2010 | Sampled | Blue | JX995357 | JX995326 |
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2010 | Sampled | Pale yellow | JX995360 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2010 | Sampled | Pale yellow | JX995361 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2010 | Sampled | Pale yellow | JX995362 | |
| Cyanea capillata | |||||||
| BS; Aarøsund Marina | 55.2574 | 9.7069 | June 2011 | Sampled | Pale orange | JX995330 | |
| BS; Aarøsund Marina | 55.2574 | 9.7069 | June 2011 | Sampled | Pale orange | JX995331 | |
| BS; Aarøsund Marina | 55.2574 | 9.7069 | June 2011 | Sampled | Pale orange | JX995332 | |
| BS; Aarøsund Marina | 55.2574 | 9.7069 | June 2011 | Sampled | Pale orange | JX995333 | |
| BS; Aarøsund Marina | 55.2574 | 9.7069 | June 2011 | Sampled | Pale orange | JX995334 | JX995327 |
| NS; Helgoland Roads | 54.1863 | 7.9000 | June 2011 | Culture | Orange | JX995335 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | June 2011 | Culture | Orange | JX995336 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | June 2011 | Culture | Orange | JX995337 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | June 2011 | Culture | Orange | JX995338 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | June 2011 | Culture | Orange | JX995339 | JX995328 |
| Icelandic waters | 67.6333 | −12.1667 | Sept 2011 | Sampled | Pale orange | JX995340 | |
| BS; Strande Marina | 54.4352 | 10.1701 | June 2009 | Sampled | Pale orange | JX995341 | |
| BS; Strande Marina | 54.4352 | 10.1701 | June 2009 | Sampled | Pale orange | JX995342 | |
| BS; Strande Marina | 54.4352 | 10.1701 | June 2009 | Sampled | Pale orange | JX995343 | |
| BS; Strande Marina | 54.4352 | 10.1701 | May 2009 | Sampled | Orange–brown | JX995344 | |
| BS; Strande Marina | 54.4352 | 10.1701 | May 2009 | Sampled | Pale orange | JX995345 | |
| BS; Strande Marina | 54.4352 | 10.1701 | May 2009 | Sampled | Orange–brown | JX995346 | |
| Aurelia aurita (outgroup taxa) | |||||||
| BS; Fehmarn Belt | Dec 2010 | JX995329 | |||||
| Sampling . | Origin . | Bell colour . | GenBank Acc. No. . | ||||
|---|---|---|---|---|---|---|---|
| Region . | Latitude . | Longitude . | Date . | COI . | 18S rDNA . | ||
| Cyanea lamarckii | |||||||
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2011 | Sampled | Colourless | JX995347 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2011 | Sampled | Colourless | JX995348 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2011 | Sampled | Colourless | JX995349 | |
| NS; German Bight | 53.7645 | 8.0629 | June 2011 | Sampled | Violet | JX995350 | |
| NS; German Bight | 53.7645 | 8.0629 | June 2011 | Sampled | Violet | JX995351 | |
| NS; German Bight | 53.7645 | 8.0629 | June 2011 | Sampled | Yellow | JX995352 | |
| NS; German Bight | 53.7645 | 8.0629 | June 2011 | Sampled | White | JX995353 | |
| NS; German Bight | 53.7645 | 8.0629 | June 2011 | Sampled | Blue | JX995354 | |
| NS; German Bight | 53.9491 | 6.7604 | June 2011 | Sampled | Yellow–white | JX995355 | |
| NS; German Bight | 54.5662 | 7.1468 | June 2011 | Sampled | Violet | JX995356 | JX995325 |
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2010 | Sampled | Blue | JX995357 | JX995326 |
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2010 | Sampled | Pale yellow | JX995360 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2010 | Sampled | Pale yellow | JX995361 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | May 2010 | Sampled | Pale yellow | JX995362 | |
| Cyanea capillata | |||||||
| BS; Aarøsund Marina | 55.2574 | 9.7069 | June 2011 | Sampled | Pale orange | JX995330 | |
| BS; Aarøsund Marina | 55.2574 | 9.7069 | June 2011 | Sampled | Pale orange | JX995331 | |
| BS; Aarøsund Marina | 55.2574 | 9.7069 | June 2011 | Sampled | Pale orange | JX995332 | |
| BS; Aarøsund Marina | 55.2574 | 9.7069 | June 2011 | Sampled | Pale orange | JX995333 | |
| BS; Aarøsund Marina | 55.2574 | 9.7069 | June 2011 | Sampled | Pale orange | JX995334 | JX995327 |
| NS; Helgoland Roads | 54.1863 | 7.9000 | June 2011 | Culture | Orange | JX995335 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | June 2011 | Culture | Orange | JX995336 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | June 2011 | Culture | Orange | JX995337 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | June 2011 | Culture | Orange | JX995338 | |
| NS; Helgoland Roads | 54.1863 | 7.9000 | June 2011 | Culture | Orange | JX995339 | JX995328 |
| Icelandic waters | 67.6333 | −12.1667 | Sept 2011 | Sampled | Pale orange | JX995340 | |
| BS; Strande Marina | 54.4352 | 10.1701 | June 2009 | Sampled | Pale orange | JX995341 | |
| BS; Strande Marina | 54.4352 | 10.1701 | June 2009 | Sampled | Pale orange | JX995342 | |
| BS; Strande Marina | 54.4352 | 10.1701 | June 2009 | Sampled | Pale orange | JX995343 | |
| BS; Strande Marina | 54.4352 | 10.1701 | May 2009 | Sampled | Orange–brown | JX995344 | |
| BS; Strande Marina | 54.4352 | 10.1701 | May 2009 | Sampled | Pale orange | JX995345 | |
| BS; Strande Marina | 54.4352 | 10.1701 | May 2009 | Sampled | Orange–brown | JX995346 | |
| Aurelia aurita (outgroup taxa) | |||||||
| BS; Fehmarn Belt | Dec 2010 | JX995329 | |||||
A total of 83 C. lamarckii medusae with live r-diameters of 14–100 mm and two medusae with r-diameter >100 mm were measured alive. All medusae then were preserved in 4% formaldehyde and were re-measured after 1 year. The colour and the presence of exumbrella papillae were re-checked after 1- and 2-years preservation. Additionally 54 C. lamarckii medusae <20 mm r-diameter were collected from Helgoland Roads (see Table I) by CALCOFI net, 500-µm mesh size and from Helgoland marina by hand net, 2-mm mesh size. These medusae were preserved in 4% formaldehyde immediately after collection. Bells were measured after 1 year preservation and living sizes were calculated by the shrinkage factor of 12.8% (see below). A total of 228 C. capillata medusae were measured and examined alive. Ten of them were re-examined after 2-years 4% formaldehyde preservation to re-check the bell colour and the persistence of the visibility of the musculature intrusions.
Rearing of young medusae
Young medusae 1–3 days after their release from the strobilae (medusae-producing polyps) were collected from polyp cultures at 5°C (C. capillata) and 10°C (C. lamarckii), respectively. The polyps were previously reared from planulae collected from medusae sampled around Helgoland (see Holst, 2012a for culturing details). Young medusae of both species were cultured for 6 weeks in 300-mL glass bottles in natural North Sea water (salinity 35 ± 2). The water was slightly aerated by using a 5-mm-diameter air tube. The tube opening leaking air bubbles near the bottom caused a weak water current and kept the young medusae in the water column. Young C. capillata medusae were fed with newly hatched Artemia salina nauplii three times per week. Dead nauplii were removed from the bottom of the bottle before each feeding and water was changed weekly. C. capillata specimens (initial number: n = 26) were reared at 10°C. Newly released C. lamarckii medusae were fed daily with suspension of mashed A. salina nauplii and sea water. Medusae were added to the food mash for 30 min and then transferred into bottles with clean sea water afterwards. In addition, a few living nauplii were added to these bottles. After the first week, young C. lamarckii medusae were fed with live A. salina nauplii as described for C. capillata. C. lamarckii was reared at 10°C and at 15°C (initial numbers: n = 31 and n = 28, respectively).
Ten medusae from each treatment were cultured in separate bottles. These medusae were transferred into small glass bowls weekly and examined under the stereomicroscope for documentation of the first appearance of papillae on the central exumbrella. The bowls were cooled with cool packs to relax the medusae during examination. Medusae in good condition and with normal symmetry (eight marginal lappets) were chosen from all cultures after 6 weeks rearing (C. capillata: 10°C, n = 21; C. lamarckii: 10°C, n = 20, 15°C, n = 12). The percentages and sizes of medusae that developed papillae on the central exumbrella were determined.
Calculations and statistics
To compare the bell size of living and preserved medusae, the r-diameter was used because it is more precise than the t-diameter (Thiel, 1960). 85 medusae >10–100 mm from field collections (C. lamarckii) and 15 medusae <10 mm reared in the laboratory were used (five C. lamarckii + ten C. capillata) to calculate the proportional relations of both diameters. The r-diameter was calculated as the percentage of the t-diameter (r-diameter*100)/t-diameter). Percentages were arcsine square root transformed before the size correlation was tested by the Spearman rank order correlation test. The percentages of shrinkage after 1-year storage in 4% formaldehyde were calculated for the same 100 medusae (100 − r-diameter preserved*100/r-diameter alive). Shrinkage percentages were arcsine square root transformed before the analysis of 10 size classes by the Spearman rank order correlation test and the Kruskal–Wallis ANOVA on ranks. Correlations of r-diameters and the maximum musculature intrusions counted in a single muscle fold were analysed by the Spearman rank order correlation test.
Molecular genetic analyses
Medusae of C. lamarckii and C. capillata were collected from the North Sea, Baltic Sea and the North Atlantic or from laboratory cultures (Table I). After documentation of the bell colour (Table I), whole specimens or parts of specimens were preserved immediately in absolute ethanol. Genomic DNA was extracted from 31 specimens using the QIAGEN DNeasy tissue kit following the manufacturer's protocol. Fragments of both fast evolving mitochondrial COI and slowly evolving nuclear ribosomal 18S rDNA were amplified using illustra PuReTaq Ready-To-Go PCR Beads (GE Healthcare) with a volume of 25 µL containing 2–4 µL DNA template and 0.5 µL of each primer (20 pmol/µL for COI and 100 pmol/µL for 18S rDNA). COI was amplified and sequenced using the primer pairs HCO2198 and LCO1490 (Folmer et al., 1994). Alternatively, HCO2198 was used together with LCOjf (Dawson, 2005b), and LCO1490 together with Nancy (Simon et al., 1994). Amplification of COI started with 94°C for 5 min followed by 38 cycles at 94°C (45 s), 42°C (45 s) and 72°C (80 s) with a final elongation at 72°C for 5–7 min. In some cases an annealing temperature of 45°C was used. The 18S rDNA fragment was amplified using the primer pairs 18A1 mod and 1800 mod (Raupach et al., 2009) with an annealing temperature of 50°C for 36 cycles. The primers F1, CF2, CR1 and R2 (Laakmann et al., 2013) were used for the sequencing reactions. PCR products were purified using QIAquick PCR Purification Kit (Qiagen) and both PCR products and purified PCR products were checked on an agarose gel (1%) with GelRed (0.1%). Sequencing was performed at Macrogen (Amsterdam, Netherlands). Sequences were edited using the software Geneious Pro version 5.4.5 created by Biomatters (available from http://www.geneious.com/) and checked for identity by BLAST in GenBank. To verify the COI nucleotide sequences, they were translated into amino acid sequences. Multiple alignments were made with the software MUSCLE (Edgar, 2004) and tree constructions as well as the calculation of pairwise genetic distances, were performed using the software MEGA (ver. 5; Tamura et al., 2011).
New sequences were deposited in GenBank (Accession numbers JX995325-57; JX995360-62; Table I). The COI sequences of C. lamarckii and C. capillata (14 and 17 sequences, respectively) were analysed together with those from the congeners C. rosea (AY902919-AY902922) and C. annaskala (AY902914-AY902917 and AY902923), as well as one C. capillata specimen from Raunefjorden, Norway (AY902911) from Dawson (Dawson, 2005a). In total, a fragment of 658 bp was analysed for 41 Cyanea spp. sequences together with Aurelia aurita as the outgroup taxa (minimum sequence length: 592 bp; variable sites of ingroup 175 bp). Neighbour Joining analysis of the COI data was performed using the K80 model (Kimura 2-parameter: equal base frequencies, one transition rate and one transversion rate; Kimura, 1980) with 1000 bootstrap replicates; pairwise genetic distances were calculated on the same substitution model.
Next to the analysis of the fast evolving mitochondrial marker COI, species discrimination was verified on the sequence divergence in a slowly evolving nuclear marker (i.e. 18S rDNA). Therefore, the 18S rDNA sequence data of C. lamarckii and C. capillata from this study (two individuals of each species) were analysed together with one C. annaskala (HM194778), one C. capillata specimen (HM194820) and Aurelia aurita as the outgroup taxa (HM194813) available in GenBank and published by Bayha et al. (Bayha et al., 2010). Maximum likelihood analysis was performed on a fragment of 1766 bp (ungapped length 1764 bp; variable sites of ingroup 6 bp) with 1000 bootstrap replicates and the best-fit evolutionary model K80 (Kimura, 1980), which yielded the best Bayesian Information Criterion score using the software jModeltest (ver. 0.1.1; Posada, 2008). Pairwise genetic distances were calculated on the K80 model as well.
RESULTS
Colouration, bell morphometry and shrinkage
In C. capillata medusae, a pale orange to dark rusty brown colour with many variations in colour intensity and bell colour patterns occurred in all specimens from field samples (Fig. 1c). In free swimming medusae with pale-orange-coloured bells, the colour was often invisible and the marginal bell lappets appeared bluish; however, the typical orange–brown colour was visible in 100% of specimens examined in the laboratory and bluish components were not found in any C. capillata bell. The orange brown colour of C. capillata was already visible in small specimens (<10 mm r-diameter) from field and culture (Fig. 1 g). All C. lamarckii medusae up to 20 mm r-diameter from field samples were colourless; 80% of medusae 20–40 mm r-diameter were colourless, only 4 of 22 specimens of this size had a pale yellow colour. All medusae >40 mm r-diameter had blue to violet or yellow coloured bells, except one colourless specimen of 55 mm r-diameter. Mixed bell colour patterns of blue and yellow components were found frequently in all sizes >40 mm r-diameter (Fig. 1d).
Bell colours had changed dramatically after 1 year storage in 4% formaldehyde. Only strong blue (in C. lamarckii) or orange (in C. capillata) colour components were still recognizable in the preserved bells. Orange and yellowish colour components, however, changed to pale brown and these colours were not useful in distinguishing between the two species. C. capillata ephyrae reared in cultures from polyps had an intense orange–brown colour (Fig. 1 g) and were easily distinguishable from the colourless C. lamarckii ephyrae (Fig. 1 h), but the intense orange colour had disappeared after 1 year storage in 4% formaldehyde.
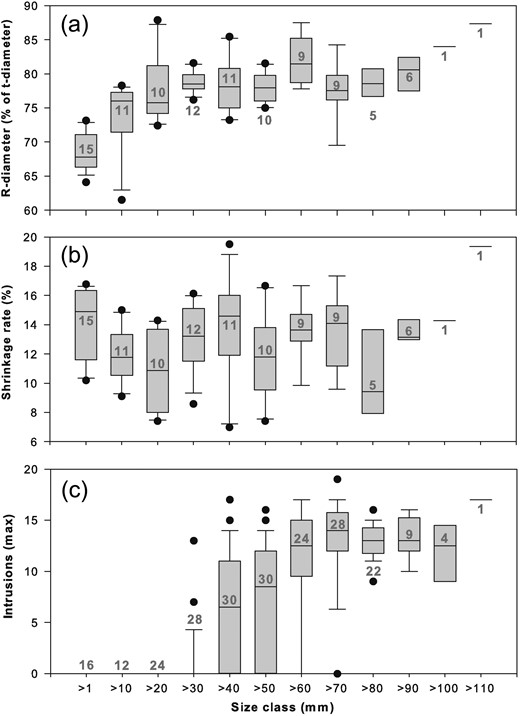
R-diameters calculated as percentages from t-diameters were slightly lower in small size classes (Fig. 2a) and increased significantly with increasing r-diameters (Spearman rank order correlation: r = 0.62, P < 0.001, n = 100). The mean shrinkage in 4% formaldehyde was 12.8% (±2.7%) for the 10 size classes (>1–10 to >90–100 mm r-diameter). Shrinkage percentages varied slightly between the size classes (Fig. 2b; ANOVA on ranks: H9 = 16.838, P = 0.051).
R-diameters, shrinkage rates and intrusions in the musculature of Cyanea medusae in different size classes (r-diameters between opposite rhopalia tips). (a) R-diameters as % of t-diameters (between opposite marginal lappet tips). (b) Shrinkage rates of medusae after 1 year storage in formaldehyde 4%. (c) Maximum numbers of intrusions found in a single muscle fold in Cyanea capillata medusae. Size classes are given in steps of 10 mm (>1 = 1–10 mm, >10 = 11–20 mm, … >110 = 111–120 mm). Box plots with medians, 25th and 75th percentiles, error bars and outlying points (black dots). Grey numbers: numbers of investigated specimens.
Musculature intrusions and exumbrella papillae
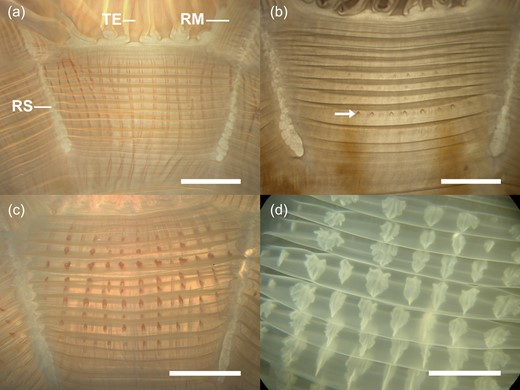
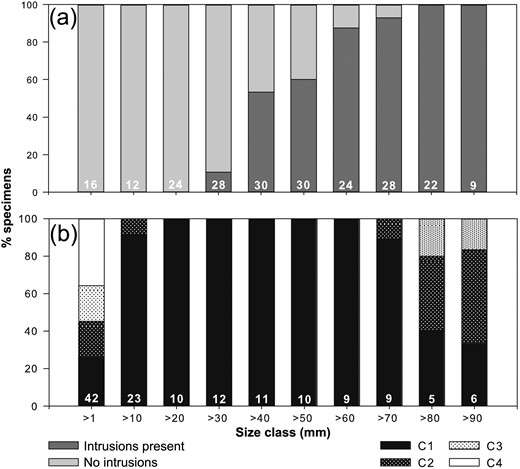
Intrusions in the adradial circular musculature (Fig. 3) were found only in C. capillata medusae >30 mm (Fig. 4a). The smallest medusa with visible intrusions was 35 mm r-diameter; in the size class >30–40 mm r-diameter, intrusions were found in only 10.7% of medusae (n = 28). Percentages of medusae with intrusions increased with the bell diameter (Fig. 4a), reaching 87.5% in medusae >60–70 mm (n = 24) and 92.9% in medusae >70–80 mm (n = 28). All medusae >80 mm had intrusions (Fig. 4a). The maximum of 19 intrusions were counted in one muscle fold in a medusa of 76 mm r-diameter (Fig. 2c). Maximal numbers counted in a single muscle fold increased with increasing r-diameters (Spearman rank order correlation: r = 0.76, P < 0.001, n = 228). Musculature intrusions were not found in any of the 85 C. lamarckii medusae examined over the size range of 14–124 mm r-diameter (19–142 mm t-diameter).
Cyanea capillata. Adradial subumbrellar muscle fields in medusae of various sizes. (a) 53 mm r-diameter, circular muscle folds (horizontally running lines) without intrusions from the radial gastric canals (vertically running lines). (b) 57 mm r-diameter, circular muscle folds with weekly developed intrusions (arrow). (c) 98 mm r-diameter, circular muscle folds with many intrusions arising from the radial gastric canals. (d) 356 mm r-diameter, circular muscle folds with pit-like-shaped intrusions. RM, radial muscle; RS, radial septum; TE, tentacle.
Presence and absence of musculature intrusions and exumbrella papillae in medusae in different size classes (r-diameters between opposite rhopalia tips) (a) Cyanea capillata (b) Cyanea lamarckii. Size classes are given in steps of 10 mm >1 = 1–10 mm, >10 = 11–20 mm, … >90 = 91–100 mm)., C1, conspicuous papillae; C2, flat or few but clearly visible papillae; C3, week and hardly visible papillae; C4, no visible. White numbers: numbers of investigated specimens.
Exumbrella papillae were not found in any C. lamarckii medusae < 3 mm r-diameter from field samples; the largest medusae in the <10 mm size class without exumbrella papillae were 5.5 mm r-diameter; 64.3% of medusae in the >1–10 mm size class had a papillose exumbrella (n = 42, Fig. 4b); of these 19.1% had inconspicuous and hardly visible papillae (C3) and 45.2% had clearly visible or conspicuous papillae (C1 and C2, Fig. 4b). All medusae in the >10–20 mm size class had conspicuous or few but clearly visible papillae (C1 and C2: 91.3% and 8.7%, n = 23, Fig. 4b). Conspicuous papillae on the central exumbrella were found in each specimen examined in the size classes from >20 to 70 mm (C1: 100%, Fig. 4b and Fig. 5a–c). Two of ten medusae examined alive in the >20–30 mm size class had spiky papillae (Fig. 5a). Most medusae >70–80 mm had prominent papillae (C1: 88.9%, n = 9, Fig. 4b), whereas one specimen had more flat but clearly visible papillae if viewed from the side (C2). In larger sizes >80–100 mm (n = 11), ∼80% had conspicuous (C1) or more flat but clearly visible papillae (C2, Figs 4b and 5d), whereas ∼20% had a very weakly papillose exumbrella (C3, Fig. 4b). The conspicuous papillae often carried nematocyst clusters (accumulations of stinging cells) at their tips (Fig. 5e), but the nematocyst clusters often disappeared when the exumbrella came into contact with the bottom of the culture vessels or with air.
Exumbrella surface of Cyanea lamarckii (a–f) and Cyanea capillata (g and h) medusae of various sizes. (a) 22 mm r-diameter, top view of the spiky exumbrella papillae. (b) 27 mm r-diameter, top view of conspicuous wart-like exumbrella papillae (c) 35 mm r-diameter, side view of conspicuous wart-like exumbrella papillae. (d) 78 mm r-diameter, side view of inconspicuous, but clearly visible exumbrella papillae. (e) nematocyst clusters at the tips of two wart-like exumbrella papillae. (f) 4.7 mm r-diameter, formalin-preserved medusa from plankton sample with small exumbrella papillae (arrow). (g and h) 22 mm and 30 mm r-diameter, typical scattered nematocyst clusters of C. capillata exumbrella. Scale bars: (a, f, h) = 1 mm; (b, c, d, g) = 2 mm; (e) = 0.2 mm.
Slight protrusions on the exumbrella appeared after 3 weeks in the young colourless C. lamarckii medusae reared from polyp cultures. The first conspicuous papillae had developed after 4 weeks in one medusa in the 10°C culture (size: 3.4 mm r-diameter) and in two medusae in the 15°C culture (size: 3.2 and 4.2 mm r-diameter, Fig. 1 h). After 6 weeks in 10°C cultures, 42.1% of 19 medusae in healthy condition had developed conspicuous papillae (4.0 ± 0.6 mm r-diameter), whereas 57.9% did not show any clearly visible exumbrella protrusions (3.1 ± 0.8 mm). At 15°C, 58.3% of 12 chosen medusae had developed clearly visible papillae (4.1 ± 1.0 mm) and 41.7% had not (3.2 ± 0.7 mm).
The degree of visibility of the exumbrella papillae (categories C1–C4) was identical in living and preserved material; specifically, not only were conspicuous papillae in living specimens still clearly visible after 1- and 2-years storage in 4% formaldehyde, but also papillae detected as flat, small or hardly visible (Fig. 5f).
Exumbrella papillae were not found in any C. capillata medusae of any size collected from field samples. The exumbrella was smooth or slightly grainy due to nematocyst clusters spread over the surface (Fig. 5g and h). No papillae developed on the exumbrella in any young medusa during 6 weeks of culture (n = 21, r-diameter = 4.40 ± 0.38 mm), even though nematocyst clusters were visible irregularly spread over the exumbrella (Fig. 1 g).
In conclusion, the character “central exumbrella papillae” is useful to distinguish C. lamarckii from C. capillata medusae >10–80 mm r-diameter. In medusae >80 mm r-diameter, “musculature intrusions” is a certain feature to distinguish C. capillata from C. lamarckii and, consequently, both morphological features together can be used to distinguish the two species in medusae >10 mm r-diameter. Living specimens <10 mm r-diameter can be distinguished by their coloration, which is colourless in C. lamarckii but intense orange in C. capillata; however, the colour disappears after preservation in formaldehyde.
Molecular genetic analyses
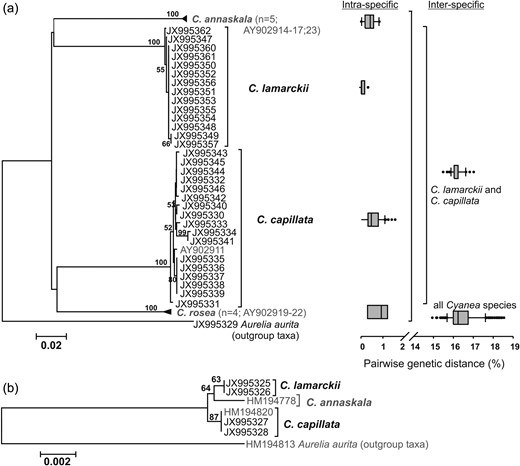
Cyanea lamarckii and C. capillata could be distinguished from each other from mitochondrial COI and nuclear ribosomal 18S rDNA sequences (Fig. 6). Based on COI, intra-specific genetic variation varied between 0 and 0.3% in C. lamarckii and between 0 and 1.5% in C. capillata and both species clades were supported by high bootstrap values of 100% (Table IIa and Fig. 6a). Inter-specific pairwise genetic distance between these two species was much higher with 15.5–17.0% (Table IIa and Fig. 6a). Consequently, the two species separate from one another by 14.0% (Fig. 6a). The C. lamarckii medusae analysed showed a large variation in bell colour, comprising blue, violet, white, different nuances of yellow or colourless, whereas in C. capillata medusa analysed, the bell colour varied from pale orange to brown orange (Table I). Among the four Cyanea species analysed, including the congeners C. annaskala and C. rosea from Dawson (Dawson, 2005a), overall intra-specific pairwise genetic distances ranged from 0 to 1.5% and inter-specific distances from 14.9 to 18.5% (Table IIa and Fig. 6a). Thus, the difference between intra- and inter-specific diversity (i.e. barcoding gap) is pronounced for all Cyanea specimens analysed (13.4% Fig. 6a), allowing the discrimination of the four species. On the 18S rDNA level, C. lamarckii, C. capillata and C. annaskala could be differentiated from each other (Fig. 6b). 18S rDNA sequences were identical within each species. Inter-specific differences ranged from 0.2 to 0.3% with the smallest difference between C. lamarckii and C. capillata (0.2%) (Table IIb and Fig. 6b).
Minimum and maximum pairwise genetic K80-distances for the investigated species in the genus Cyanea based on (a) mitochondrial COI and (b) nuclear 18S rDNA
| . | C. annaskala . | C. capillata . | C. lamarckii . | C. rosea . |
|---|---|---|---|---|
| (a) | ||||
| C. annaskala | 0.0–0.8 | |||
| C. capillata | 16.4–18.4 | 0.0–1.5 | ||
| C. lamarckii | 15.9–17.0 | 15.5–17–0 | 0.0–0.3 | |
| C. rosea | 17.4–18.5 | 14.9–16.5 | 15.6–16.2 | 0.2–1.3 |
| (b) | ||||
| C. annaskala | 0 | |||
| C. capillata | 0.3 | 0 | ||
| C. lamarckii | 0.2 | 0.2 | 0 | |
| Aurelia aurita | 2.9 | 2.8 | 2.8 |
| . | C. annaskala . | C. capillata . | C. lamarckii . | C. rosea . |
|---|---|---|---|---|
| (a) | ||||
| C. annaskala | 0.0–0.8 | |||
| C. capillata | 16.4–18.4 | 0.0–1.5 | ||
| C. lamarckii | 15.9–17.0 | 15.5–17–0 | 0.0–0.3 | |
| C. rosea | 17.4–18.5 | 14.9–16.5 | 15.6–16.2 | 0.2–1.3 |
| (b) | ||||
| C. annaskala | 0 | |||
| C. capillata | 0.3 | 0 | ||
| C. lamarckii | 0.2 | 0.2 | 0 | |
| Aurelia aurita | 2.9 | 2.8 | 2.8 |
Minimum and maximum pairwise genetic K80-distances for the investigated species in the genus Cyanea based on (a) mitochondrial COI and (b) nuclear 18S rDNA
| . | C. annaskala . | C. capillata . | C. lamarckii . | C. rosea . |
|---|---|---|---|---|
| (a) | ||||
| C. annaskala | 0.0–0.8 | |||
| C. capillata | 16.4–18.4 | 0.0–1.5 | ||
| C. lamarckii | 15.9–17.0 | 15.5–17–0 | 0.0–0.3 | |
| C. rosea | 17.4–18.5 | 14.9–16.5 | 15.6–16.2 | 0.2–1.3 |
| (b) | ||||
| C. annaskala | 0 | |||
| C. capillata | 0.3 | 0 | ||
| C. lamarckii | 0.2 | 0.2 | 0 | |
| Aurelia aurita | 2.9 | 2.8 | 2.8 |
| . | C. annaskala . | C. capillata . | C. lamarckii . | C. rosea . |
|---|---|---|---|---|
| (a) | ||||
| C. annaskala | 0.0–0.8 | |||
| C. capillata | 16.4–18.4 | 0.0–1.5 | ||
| C. lamarckii | 15.9–17.0 | 15.5–17–0 | 0.0–0.3 | |
| C. rosea | 17.4–18.5 | 14.9–16.5 | 15.6–16.2 | 0.2–1.3 |
| (b) | ||||
| C. annaskala | 0 | |||
| C. capillata | 0.3 | 0 | ||
| C. lamarckii | 0.2 | 0.2 | 0 | |
| Aurelia aurita | 2.9 | 2.8 | 2.8 |
Molecular genetic analyses of Cyanea lamarckii and Cyanea capillata. (a) Neighbour Joining analysis of 658 bp-fragment of cytochrome c oxidase subunit I based on the K80 model (Kimura, 1980) with 1000 bootstrap replicates including Cyanea rosea and Cyanea annaskala and one C. capillata specimen (in grey) from Dawson (Dawson, 2005a). Pairwise genetic distances on intra- and inter-specific levels are given as box plots. Boxes include 50% of the data set, the whiskers 10 and 90%, and black dots depict the outliers. (b) Maximum likelihood analysis of 1766 bp-fragment of 18S rDNA based on the K80 model (Kimura, 1980) with 1000 bootstrap replicates including C. annaskala and one C. capillata specimen (in grey) from Bayha et al. (Bayha et al., 2010).
DISCUSSION
Our integrated taxonomic approach comprising morphological examinations and molecular genetic analyses that included different life history stages revealed the two different Cyanea species, C. lamarckii and C. capillata. The complex taxonomic history of southeastern Australian Cyanea species was reviewed by Dawson (Dawson, 2005a). Northwest Atlantic Cyanea species were investigated by Bayha (Bayha, 2005); however, the number and the inter-specific differentiation of Cyanea species in the North Atlantic is still an unsolved question. Not all of the Cyanea species from the North Atlantic mentioned by various authors are currently regarded as valid, but recent molecular studies indicate higher species richness in Cyanea than estimated by authors who synonymized many taxa (see Bayha et al., 2010). A comprehensive revision of the genus Cyanea, as recently provided for the genus Chrysaora (Morandini and Marques, 2010), is needed to assess the validity of described Cyanea species. Haeckel (Haeckel, 1880) and Agassiz (Agassiz, 1862) regarded C. lamarckii as a valid species with an overlapping distribution with C. capillata in the northeast Atlantic. They considered at least two additional species from the northwest Atlantic coast as valid and distinguishable species: Cyanea fulva, Cyanea arctica [probably including C. fulva as a colour variation of C. arctica after Haeckel (1880)], and Cyanea versicolor. Later C. capillata was considered to exhibit a global distribution including the North Atlantic and North Pacific (e.g. Kramp, 1961; Russell, 1970), and thus several Cyanea species were synonymized with C. capillata, e.g. C. arctica, C. versicolor and C. annaskala (Kramp, 1961). This was rebutted by Dawson (Dawson, 2005a) who found Cyanea species from Australian waters to be morphologically and genetically different from C. capillata collected in the North Sea. Regarding the western Atlantic Cyanea species classified as varieties of C. capillata (Mayer, 1910; Kramp, 1961; Russell, 1970), Brewer (Brewer, 1991) reported that at least two Cyanea species can be morphologically distinguished on the North American coast and Bayha (Bayha, 2005) described three novel molecularly distinguishable species of Cyanea in US Atlantic waters. Our study now complements these descriptions of the western Atlantic Cyanea species with detailed morphological and molecular data for the eastern Atlantic Cyanea species.
In accordance with previous studies, our investigations confirm the intrusions in the subumbrellar musculature as a diagnostic feature for C. capillata from the northeast Atlantic (Russell, 1970, 1978); however, our study demonstrates that this feature is not consistent in specimens <80 mm r-diameter. Musculature intrusions were also described in Cyanea species from the Pacific (see Kishinouye, 1910 for Cyanea citrea and Cyanea purpurea, see Dawson, 2005a for Cyanea rosea). Detailed descriptions and drawings of musculature intrusions of C. capillata branching from the gastric canals into the muscle folds are given by Russell (Russell, 1970, Text-fig. 57). It was suggested that the function of the intrusions arising from the gastric canals may be to optimize nutrient transport to the musculature (Stiasny and Maaden, 1943).
Exumbrella papillae were described for the northeast Atlantic C. lamarckii (Östergren, 1909 as Cyanea palmstruchii; Russell, 1970) as well as for the southeast Australian C. rosea (Dawson, 2005a) and young specimens of C. fulva of the northeast Atlantic coast (Agassiz, 1862; Fewkes, 1881; Mayer, 1910). Spiky exumbrella papillae, as described for two C. lamarckii specimens of ∼20 mm r-diameter in the present study (Fig. 5a), seem to appear typically in small Cyanea spp. with papillose exumbrellas (Agassiz, 1862; Fewkes, 1881; Östergren, 1909; Mayer, 1910). Dong et al. (Dong et al., 2008) described nematocyst batteries on the central exumbrella that were present in C. nozakii and C. purpurea, but absent in C. capillata and C. ferruginea from Chinese waters; however, the authors did not mention if these nematocyst batteries were located on a smooth or papillose exumbrella. Our examinations and rearing experiments of young medusae confirmed the absence of exumbrella papillae in accordance with previous descriptions of young C. capillata (Östergren, 1909; Russell, 1970). In contrast, exumbrella papillae already appear in young C. lamarckii <10 mm r-diameter (Östergren, 1909 as C. palmstruchii; present study). In the first early life-cycle description of C. lamarckii by Delap (Delap, 1905), the appearance of exumbrella papillae was not mentioned in the text, but a papillose exumbrella is represented in her drawing of a young medusa of ∼7 mm bell diameter (Delap, 1905, plate I.3).
Our study confirms that the papillose exumbrella is a useful diagnostic feature to distinguish C. lamarckii from C. capillata from the northeast Atlantic in sizes <80 mm r-diameter. In larger sizes (>80 mm r-diameter), the intrusions in the circular subumbrellar musculature are a certain diagnostic feature to identify C. capillata in this region. The intrusions in large medusae are usually visible by eye as a typical pattern in the subumbrellar musculature (Russell, 1970; Holst, 2012a; Fig. 1f), which makes this feature useful for quick species identification in the field. Musculature intrusions were not found in any C. lamarckii medusae investigated in the present study (up to 142 mm in total diameter), but it should be noted that small numbers of intrusions may exceptionally occur in large C. lamarckii medusae as mentioned by Russell (Russell, 1970). The feature “central exumbrella papillae” allows quick identification of C. lamarckii medusae >20–80 mm r-diameter. In small individuals or if uncertain, medusae can be examined with a stereomicroscope to identify the exumbrella papillae in C. lamarckii or the smooth exumbrella surface and the intrusions in the musculature in C. capillata, respectively. In contrast to this relatively easy method, the application of tentacle numbers as a diagnostic feature has many difficulties and is much more time consuming (Thiel, 1962). Exumbrella papillae are also typical for medusae of the species Pelagia noctiluca (Russell, 1970, 1978) that occurs in the Mediterranean and the North Atlantic (Stopar et al., 2010); however, specimens of Pelagidae can be distinguished from Cyaneidae because in the latter, the tentacles arise at a distance from the bell margin on the subumbrella, whereas the tentacles of Pelagidae arise at the bell margin (Bayha and Dawson, 2010; Holst, 2012a).
In addition to these differences in morphology and genetics, the two Cyanea species from the northeast Atlantic differ in their early life-stages, for example, planulae and newly released ephyrae are smaller in size in C. lamarckii than in C. capillata (Holst, 2012a). As demonstrated by laboratory experiments, the polyps of the two species have different eco-physiological demands, e.g. warm winter temperatures of 15°C supported the production of young medusae in C. lamarckii, whereas C. capillata polyps produced medusa at colder temperatures of 10 and 5°C, only (Holst, 2012b). Planulae of both species collected in the German Bight tolerated remarkable salinity reduction with planula settlement at salinity 20 and strobilation at the even lower salinity of 12 (Holst and Jarms, 2010). However, C. capillata developed into polyps directly after settlement whereas in C. lamarckii most planulae encysted at all salinities from 32 to 20 (Holst and Jarms, 2007, 2010). In addition to these differences, the morphologically identical nematocysts of the two species differ in size and distribution in the medusa tissues, even if specimens of similar diameters are compared (Östman and Hydman, 1997). Moreover, the haemolytic potency of nematocysts is lower in C. lamarckii (Helmholz et al., 2007) than in C. capillata nematocysts, which contain a highly effective toxin that causes a painful sting in humans (Heeger et al., 1992; Lassen et al., 2010).
Shrinkage, the loss of colour and diagnostic features, and distortions of the body proportions due to preservatives are general problems in morphological investigations on gelatinous animals such as scyphozoan and hydrozoan medusae (Laakmann and Holst, 2013). Although highly poisonous, formaldehyde solutions are preferred to the less poisonous ethanol in order to preserve the diagnostic features of their delicate bodies (Schuchert, 2012). Some tissue parts should also be preserved in absolute ethanol to allow parallel DNA extraction and morphological studies of the same individuals.
Our study provides extensive descriptions on shrinkage, colour changes and durability of diagnostic features in Cyanea spp. after storage in 4% formaldehyde.
The shrinkage rate determined of ∼13% could help calculate the original size of formalin-preserved Cyanea spp. or other semaeostome medusae stored in museum collections. For accurate bell measurements, the diameters between opposite rhopalia tips are useful. Detection of the rhopalia tips is more reliable than the tips of roundish marginal lappets which are, often damaged or contracted (Thiel, 1960). Importantly, the percentages of the r-diameters are not constant, but increase proportionally to the t-diameters with increasing size (Fig. 2a), indicating that the marginal lappets surrounding the rhopalia are proportionally smaller in larger medusae. Some diagnostic features of northeast Atlantic scyphomedusae are already identifiable in young developmental stages and remain in formaldehyde-preserved samples (Holst, 2012a). The most obvious morphological character of the Cyanidae is the arrangement of the tentacles in eight clusters inserting on the subumbrella (Bayha and Dawson, 2010), in contrast to the tentacles inserting at the bell margin in other semaeostome scyphomedusae, e.g. in the Pelagidae and Ulmaridae (Holst, 2012a). The present study confirms that the features “intrusions in the musculature” and “central exumbrella papillae” are additional diagnostic features that remain in formalin-preserved specimens of different sizes, whereas the colour changes or disappears after preservation. For researchers with extensive experience, the more orange–brown colour of C. capillata may be distinguishable from the light to pale yellow in the yellow-coloured C. lamarckii specimens, but the colour is definitely not useful as a diagnostic feature in preserved material.
The two species, C. lamarckii and C. capillata, can clearly be distinguished from one another from both nuclear and on mitochondrial gene fragments. Because the specimens analysed comprised different life history stages, as well as showing a variety in bell colouration, our study underlines the power of molecular genetic tools to be integrated in morphological taxonomic studies. The inter-specific genetic variations of 14.9–18.5% on the COI level observed between Cyanea congeners in this study are comparable with those found between other scyphozoan congeners (e.g. Aurelia: Dawson and Jacobs, 2001; Cassiopea: Holland et al., 2004; Drymonema:Bayha and Dawson, 2010). Also on intra-specific level, the genetic variation was comparable with that of other scyphozoans (e.g. Ortman et al., 2010). Thus, COI qualifies for successful valid species identification and discrimination of Scyphozoa with well pronounced barcoding gap and divergences similar to those of other metazoans (Huang et al., 2008).
Compared with mitochondrial gene fragments, the inter-specific variation in the more slowly evolving nuclear 18S rDNA gene fragment was much lower. However, even this slight difference in the highly conserved 18S rDNA verified the state of the two different species. This low genetic variability on the 18S rDNA level within the Cyanea genus is in accordance with similar findings in species of the genus Aurelia (Ki et al., 2009). At the intra-specific level, sequence variation was absent in the Cyanea species investigated which is, in general, a consequence of concerted evolution (e.g. Eickbush and Eickbush, 2007).
The analysis of nucleotides, which are independent of the life stage, is a powerful tool especially for the jellyfish life-stages with limited or stage-specific morphological characters. For example, the identification of diagnostic features in polyps and ephyra stages is often difficult and requires labour-intensive examinations (Holst, 2012a). Thus, molecular genetic tools can support the findings of morphological differences and variances within and between species, as well as highlight and identify those diagnostic morphological characters. Together with previous morphological and molecular descriptions on northeast Atlantic jellyfish in different developmental stages, the present descriptions can help to analyse the age structure of jellyfish blooms and allow identification of young medusae. In turn, this could lead to conclusions regarding their point of origin of medusae producing benthic polyps (Holst, 2012a). Together with studies of the population genetic structure of jellyfish species (e.g. Lee et al., 2013), this may promote investigation of the reproductive biology of benthic stages and to ascertain the geographical origins of jellyfish outbreaks. This knowledge is fundamentally important to understand the causes of jellyfish fluctuations and to improve future management of jellyfish blooms.
FUNDING
This work was partly supported by the Federal Ministry of Education and Research (Grant No. 03F0499A) and the Land Niedersachsen.
ACKNOWLEDGEMENTS
We thank the Biologische Anstalt Helgoland (Alfred Wegener Institute) and the Biocenter Grindel and Zoological Museum (University of Hamburg) for providing the research facilities. We thank Rebekka Schüller and Magreth Krüss (AWI Helgoland) for their help in medusa sampling and Anna Meyer-Löbbecke for her assistance in examinations and culture experiments. We are grateful to J. E. Purcell for English language revision and to the anonymous reviewers for helpful comments to improve the manuscript.
REFERENCES
Author notes
Corresponding editor: Marja Koski