-
PDF
- Split View
-
Views
-
Cite
Cite
Pär Stattin, Erik Holmberg, Jan-Erik Johansson, Lars Holmberg, Jan Adolfsson, Jonas Hugosson, on behalf of the National Prostate Cancer Register (NPCR) of Sweden, Outcomes in Localized Prostate Cancer: National Prostate Cancer Register of Sweden Follow-up Study, JNCI: Journal of the National Cancer Institute, Volume 102, Issue 13, 7 July 2010, Pages 950–958, https://doi.org/10.1093/jnci/djq154
Close - Share Icon Share
Abstract
Treatment for localized prostate cancer remains controversial. To our knowledge, there are no outcome studies from contemporary population-based cohorts that include data on stage, Gleason score, and serum levels of prostate-specific antigen (PSA).
In the National Prostate Cancer Register of Sweden Follow-up Study, a nationwide cohort, we identified 6849 patients aged 70 years or younger. Inclusion criteria were diagnosis with local clinical stage T1–2 prostate cancer from January 1, 1997, through December 31, 2002, a Gleason score of 7 or less, a serum PSA level of less than 20 ng/mL, and treatment with surveillance (including active surveillance and watchful waiting, n = 2021) or curative intent (including radical prostatectomy, n = 3399, and radiation therapy, n = 1429). Among the 6849 patients, 2686 had low-risk prostate cancer (ie, clinical stage T1, Gleason score 2-6, and serum PSA level of <10 ng/mL). The study cohort was linked to the Cause of Death Register, and cumulative incidence of death from prostate cancer and competing causes was calculated.
For the combination of low- and intermediate-risk prostate cancers, calculated cumulative 10-year prostate cancer–specific mortality was 3.6% (95% confidence interval [CI] = 2.7% to 4.8%) in the surveillance group and 2.7% (95% CI = 2.1% to 3.45) in the curative intent group. For those with low-risk disease, the corresponding values were 2.4% (95% CI = 1.2% to 4.1%) among the 1085 patients in the surveillance group and 0.7% (95% CI = 0.3% to 1.4%) among the 1601 patients in the curative intent group. The 10-year risk of dying from competing causes was 19.2% (95% CI = 17.2% to 21.3%) in the surveillance group and 10.2% (95% CI = 9.0% to 11.4%) in the curative intent group.
A 10-year prostate cancer–specific mortality of 2.4% among patients with low-risk prostate cancer in the surveillance group indicates that surveillance may be a suitable treatment option for many patients with low-risk disease.
Treatment of localized prostate cancer is controversial.
Retrospective study in a nationwide cohort of patients with localized prostate cancer who were 70 years or younger. These patients received surveillance (including active surveillance and watchful waiting) or treatment with curative intent (including radical prostatectomy and radiation therapy). A subgroup of patients with low-risk prostate cancer was also studied. The cumulative incidence of death from prostate cancer and competing causes was calculated.
For the combination of low- and intermediate-risk prostate cancers, calculated cumulative 10-year prostate cancer–specific mortality was 3.6% in the surveillance group and 2.7% in the curative intent group. For those with low-risk disease, the corresponding values were 2.4% in the surveillance group and 0.7% in the curative intent group. The 10-year risk of dying from competing causes was 19.2% in the surveillance group and 10.2% in the curative intent group.
The low 10-year prostate cancer–specific mortality observed among patients with low-risk prostate cancer in the surveillance group indicates that surveillance may be a suitable treatment option for many patients with low-risk disease.
The observational design resulted in a strong selection bias in which a higher proportion of healthy patients with prostate cancer with adverse factors were assigned to radical prostatectomy than to surveillance. No information was available on tumor extent in core biopsy specimens, serum PSA levels after the date of diagnosis, or progression to metastatic disease. The median follow-up time was limited to 8.2 years.
From the Editors
There is a growing concern for overtreatment of localized prostate cancer because patients currently have earlier-stage disease than before the introduction of prostate-specific antigen (PSA) ( 1–3 ). Therefore, active surveillance (ie, deferred curative treatment for low-risk prostate cancer until the perceived disease progression) has attracted increasing attention ( 4 , 5 ). Some information has been published on intermediate- and long-term outcomes of surveillance from patients at single institutions ( 6–9 ), but, to the best of our knowledge, since the introduction of PSA, no results have been published from outcome studies of nationwide population-based cohorts that include data on clinical stage, Gleason score, serum levels of PSA, and primary treatment.
After a median follow-up time of 9 years, the European Randomized Study of Screening for Prostate Cancer (ERSPC) reported lower prostate cancer–specific death rates (0.29%) in the screening arm than in the control arm (0.36%) (relative risk [RR] = 0.80, 95% confidence interval [CI] = 0.67 to 0.95) ( 10 ). There was considerable overdiagnosis and overtreatment in the screening arm because 1068 men had to be screened and 48 men had to undergo curative treatment to save one life. After 11 years of follow-up, the Scandinavian Prostate Cancer Group study number 4 (SPCG-4), the only sufficiently large randomized clinical trial on curative treatment for localized prostate cancer to date, reported a prostate cancer–specific mortality of 12.5% in the prostatectomy arm and of 17.9% in the control arm (RR = 0.65, 95% CI = 0.45 to 0.94) ( 11 ). It should be noted that patients in the SPCG-4 trial had advanced prostate cancer by current standards and that watchful waiting, not active surveillance, was used in the control arm. Finally, a study of data from the Surveillance, Epidemiology, and End Results (SEER) program in the United States reported a 10-year prostate cancer–specific mortality of 8.3% (which was substantially lower than the value reported in the control arm of the SPCG-4 trial) among elderly patients with localized prostate cancer who were treated conservatively ( 12 ).
The aim of this nationwide population-based cohort study was to assess prostate cancer mortality and risk of death from competing causes in patients in the National Prostate Cancer Register (NPCR) of Sweden Follow-up Study (hereafter the NPCR Follow-up Study) who had low- or intermediate-risk prostate cancer and who were treated with surveillance, radical prostatectomy, or radiation therapy as received in routine clinical practice in Sweden from January 1, 1997, through December 31, 2002. Data were collected on clinical stage, Gleason score, serum PSA level, comorbidity, and socioeconomic status.
Participants and Methods
The Swedish Cancer Register and the NPCR of Sweden
Data on all cancer patients in Sweden must be submitted to the Swedish Cancer Register by law. The capture rate of this register has been estimated to be approximately 98% for solid tumors among patients younger than 75 years ( 13 , 14 ). Currently, 98% of all patients with newly diagnosed (ie, incident) prostate cancer in the Swedish Cancer Register are also registered in the NPCR, which contains data on TNM stage, tumor differentiation, serum PSA levels at the time of diagnosis, and primary treatment within 6 months of the date of diagnosis ( 15–18 ). During the study period, conservative treatment (coded as “expectancy” in NPCR) included both active surveillance (ie, a strategy for delivering curative treatment when progression occurred) and watchful waiting (ie, a strategy for administering hormonal treatment when symptomatic progression occurred).
Data Extraction for the Follow-up Study
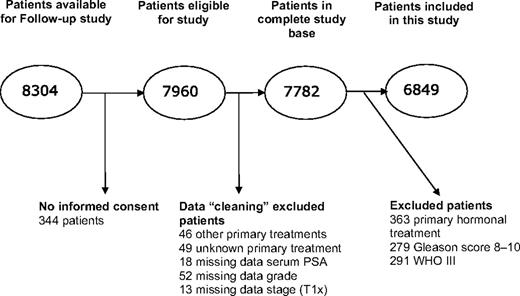
The Follow-up Study was an observational study in the NPCR, with the aim of assessing outcome among patients with localized prostate cancer who were diagnosed after PSA testing had become prevalent in Sweden and for whom curative treatment may have been indicated. Inclusion criteria were registration in NPCR from January 1, 1997 (January 1, 1998, in one region), through December 31, 2002; age 70 years or younger at the date of diagnosis; local tumor stage T1–2 (in NPCR, clinical local tumor stage T2 is reported without any further subclassification into T2a, b, c); no signs of lymph node metastases (Nx or N0) or bone metastasis (Mx or M0); and serum PSA levels of less than 20 ng/mL ( 19 ). In total, 8304 patients fulfilled these criteria and 7960 accepted inclusion to the study ( Figure 1 ). Verification of eligibility, data on treatment, and the date that surveillance was terminated were extracted from medical records by research nurses in each of six health-care regions in Sweden at a median time of 4 years after the date of diagnosis. We excluded 363 patients who had received primary hormonal treatment, and because we wanted to study outcome in patients for whom surveillance is an accepted treatment option, we further excluded 570 patients with poorly differentiated tumors (Gleason score 8–10 or World Health Organization grade III). The final study set consisted of 6849 patients, including 2686 who had low-risk prostate cancer (ie, clinical stage T1, Gleason score 2-6 or World Health Organisation grade I-II, and serum PSA level of <10 ng/mL). Treatment, which was surveillance (n = 2021), or with curative intent including radical prostatectomy (n = 3399) or radiation therapy (n = 1429), was assigned in clinical practice at the discretion of treating physician and patient. There was no uniform protocol that defined indications for surveillance, follow-up procedures, or criteria for initiation of deferred treatment in use at that time in Sweden.
Identification and exclusion of men in the National Prostate Cancer Register (NPCR) of Sweden Follow-up Study. The complete database was defined as patients remaining after cleaning of the data set, before exclusion of patients who received hormonal treatment or had poorly differentiated tumors. Data cleaning is exclusion of men with primary treatments other than surveillance, prostatectomy, or radiotherapy; unknown primary treatment; or missing data on PSA, grade, or stage. PSA = prostate-specific antigen; T1x = primary tumor cannot be assessed; WHO = World Health Organization.
By use of the unique 10-digit Swedish person identity number, we linked the NPCR Follow-up Study database to several other national databases. Complete follow-up regarding vital status from the date of diagnosis until December 31, 2008, was obtained by linkage to the Swedish Population Register, and assessment of cause of death was obtained by linkage to the Cause of Death Register up to December 31, 2007. Cause of death for patients who died from December 31, 2007, through December 31, 2008, was assessed by review of death certificates not yet entered into the Cause of Death Register by a research nurse. If there was uncertainty, then a urologist was consulted. Death was attributed to prostate cancer when prostate cancer was coded as “underlying cause of death.” We used the socioeconomic status from the Census Databases of 1960–1990, which classified patients into lower socioeconomic status (including blue-collar and low-level white-collar workers), higher socioeconomic status (including intermediate- and high-level white-collar workers and the self-employed), and unknown or missing socioeconomic status ( 20 , 21 ). We used the last socioeconomic status registered because many men had retired at the date of diagnosis. In the analysis, we also included a Charlson comorbidity index constructed on grouping of International Classification of Diseases ( ICD ) codes as described previously ( 22 , 23 ). We based the classification on data in the Patient Register, which contains data on discharge diagnoses, surgical procedures, and date of admission and discharge, which is updated annually and which has a capture rate for somatic inpatient care of virtually 100% since 1987 ( 24 ). The Research Ethics Committee of Gothenburg University approved of the study in which an opt-out consent was distributed to all study subjects.
Statistical Analysis
The χ 2 test and the t test were used to test the hypothesis that there was no difference in the distribution of the patient characteristics by treatment groups. All statistical tests were two-sided. The main endpoints in the analysis were death from prostate cancer, death from competing causes, and death from all causes. The endpoint for comparisons between observed and expected mortality was death from all causes, as estimated by use of population death rates from the entire Swedish population matched to the study population by age and calendar year.
Cumulative incidence of mortality ( 25 , 26 ) and relative risk were used to measure associations with the main endpoints. The Pepe and Mori test ( 27 ) was used to test the hypothesis that there was no difference in the cumulative incidence of mortality between treatment groups. The relative risk was estimated by use of the Cox proportional hazard model and competing-risks regression models according to the method of Fine and Gray ( 28 ). The proportional hazard assumption was tested with Schoenfeld residuals, and we found no violation of the assumption of proportional hazards ( 29 ).
Because treatment effects may be larger among patients with intermediate-risk prostate cancer and because surveillance is particularly advocated for low-risk cancers ( 4–6 , 30 ), we also analyzed results by risk category. The low-risk category was defined as clinical local stage T1a, b, or c and Gleason score 2-6 or World Health Organization grade I-II and a serum PSA level of less than 10 ng/mL. The intermediate-risk category was defined as tumor stage T2 or Gleason score of 7 or serum PSA of 10 ng/mL or higher. The Stata statistical software was used for all statistical analyses ( 31 ). Adjustments in analyses were made for categories of comorbidity (Charlson index of 0–1 or ≥2), socioeconomic group (higher or lower), risk group (low or intermediate), and age as a continuous variable.
Results
Among the 6849 patients, surveillance was started in 2021 (29.5%), radical prostatectomy was performed in 3399 (49.6%), and radiation therapy was delivered to 1429 (20.9%) ( Table 1 ). A larger proportion of patients with low-risk tumors (40.4%) were treated with surveillance than patients with intermediate-risk tumors (22.5%). After a median follow-up time of 4 years, 692 (34%) of the 2021 patients on surveillance had received deferred treatment, which was radical prostatectomy for 277 men, radiation therapy for 207 men, and hormonal therapy for 208 men. More than 90% of all patients had a Charlson index of 0 or 1 at the time of diagnosis. Surveillance was more common in patients with high comorbidity and was initiated in 1807 (28.4%) of the 6347 patients with a Charlson index of 0–1 and in 220 (43.8%) of the 502 patients with a Charlson index 2 or higher. A slightly higher proportion of the 2563 patients with lower socioeconomic status received surveillance, 804 (31.4%), than of the 4125 patients with higher socioeconomic status, 1134 (27.5%).
Recruitment period, age at diagnosis, tumor characteristics, comorbidity, and socioeconomic index for 6849 patients with prostate cancer: the National Prostate Cancer Register (NPCR) of Sweden Follow-up Study *
| Characteristic | Surveillance group (n = 2021) | Radical prostatectomy group (n = 3399) | Radiation therapy group (n = 1429) | Total cohort |
| Year of recruitment † , No. (%) | ||||
| 1997–1998 | 569 (41.4) | 584 (42.5) | 221 (16.1) | 1374 |
| 1999–2000 | 667 (28.6) | 1148 (49.3) | 515 (22.1) | 2330 |
| 2000–2001 | 785 (25.0) | 1667 (53.0) | 693 (22.0) | 3145 |
| Age at diagnosis | ||||
| Age group, No. (%) | ||||
| <60 y | 299 (16.3) | 1224 (66.7) | 313 (17.0) | 1836 |
| 60–64 y | 519 (25.5) | 1098 (53.9) | 419 (20.6) | 2036 |
| 65–70 y | 1203 (40.4) | 1077 (36.2) | 697 (23.4) | 2977 |
| Mean, y (SD) | 64.7 (4.6) | 61.2 (5.3) | 63.4 (4.9) | |
| Clinical local T stage, No. (%) | ||||
| T1a | 331 (83.0) | 51 (12.8) | 17 (4.3) | 399 |
| T1b | 91 (54.8) | 46 (27.7) | 29 (17.5) | 166 |
| T1c | 1021 (29.6) | 1805 (52.3) | 624 (18.1) | 3450 |
| T2 | 578 (20.4) | 1497 (52.8) | 759 (26.8) | 2834 |
| Tumor differentiation, No. (%) | ||||
| Gleason score 2–6 or WHO I or II | 1928 (32.8) | 2798 (47.6) | 1149 (19.6) | 5875 |
| Gleason score 7 | 93 (9.5) | 601 (61.7) | 280 (28.7) | 974 |
| Serum PSA level | ||||
| Category, No. (%) | ||||
| 0–4 ng/mL | 422 (48.8) | 343 (39.7) | 100 (11.6) | 865 |
| 4–10 ng/mL | 1049 (26.9) | 2064 (52.9) | 787 (20.2) | 3900 |
| 10–20 ng/mL | 550 (26.4) | 992 (47.6) | 542 (26.0) | 2084 |
| Mean, ng/mL (SD) | 7.6 (4.4) | 8.2 (3.9) | 9.3 (4.2) | |
| Risk category ‡ , No. (%) | ||||
| Low | 1085 (40.4) | 1227 (45.7) | 374 (13.9) | 2686 |
| Intermediate | 936 (22.5) | 2172 (52.2) | 1055 (25.3) | 4163 |
| Charlson comorbidity index, No. (%) | ||||
| 0–1 | 1801 (28.4) | 3233 (50.9) | 1313 (20.7) | 6347 |
| ≥2 | 220 (43.8) | 166 (33.1) | 116 (23.1) | 502 |
| Socioeconomic index, No. (%) | ||||
| Low | 804 (31.4) | 1199 (46.8) | 560 (21.8) | 2563 |
| High | 1134 (27.5) | 2152 (52.2) | 839 (20.3) | 4125 |
| Undefined or missing | 83 (51.6) | 48 (29.8) | 30 (18.6) | 161 |
| Characteristic | Surveillance group (n = 2021) | Radical prostatectomy group (n = 3399) | Radiation therapy group (n = 1429) | Total cohort |
| Year of recruitment † , No. (%) | ||||
| 1997–1998 | 569 (41.4) | 584 (42.5) | 221 (16.1) | 1374 |
| 1999–2000 | 667 (28.6) | 1148 (49.3) | 515 (22.1) | 2330 |
| 2000–2001 | 785 (25.0) | 1667 (53.0) | 693 (22.0) | 3145 |
| Age at diagnosis | ||||
| Age group, No. (%) | ||||
| <60 y | 299 (16.3) | 1224 (66.7) | 313 (17.0) | 1836 |
| 60–64 y | 519 (25.5) | 1098 (53.9) | 419 (20.6) | 2036 |
| 65–70 y | 1203 (40.4) | 1077 (36.2) | 697 (23.4) | 2977 |
| Mean, y (SD) | 64.7 (4.6) | 61.2 (5.3) | 63.4 (4.9) | |
| Clinical local T stage, No. (%) | ||||
| T1a | 331 (83.0) | 51 (12.8) | 17 (4.3) | 399 |
| T1b | 91 (54.8) | 46 (27.7) | 29 (17.5) | 166 |
| T1c | 1021 (29.6) | 1805 (52.3) | 624 (18.1) | 3450 |
| T2 | 578 (20.4) | 1497 (52.8) | 759 (26.8) | 2834 |
| Tumor differentiation, No. (%) | ||||
| Gleason score 2–6 or WHO I or II | 1928 (32.8) | 2798 (47.6) | 1149 (19.6) | 5875 |
| Gleason score 7 | 93 (9.5) | 601 (61.7) | 280 (28.7) | 974 |
| Serum PSA level | ||||
| Category, No. (%) | ||||
| 0–4 ng/mL | 422 (48.8) | 343 (39.7) | 100 (11.6) | 865 |
| 4–10 ng/mL | 1049 (26.9) | 2064 (52.9) | 787 (20.2) | 3900 |
| 10–20 ng/mL | 550 (26.4) | 992 (47.6) | 542 (26.0) | 2084 |
| Mean, ng/mL (SD) | 7.6 (4.4) | 8.2 (3.9) | 9.3 (4.2) | |
| Risk category ‡ , No. (%) | ||||
| Low | 1085 (40.4) | 1227 (45.7) | 374 (13.9) | 2686 |
| Intermediate | 936 (22.5) | 2172 (52.2) | 1055 (25.3) | 4163 |
| Charlson comorbidity index, No. (%) | ||||
| 0–1 | 1801 (28.4) | 3233 (50.9) | 1313 (20.7) | 6347 |
| ≥2 | 220 (43.8) | 166 (33.1) | 116 (23.1) | 502 |
| Socioeconomic index, No. (%) | ||||
| Low | 804 (31.4) | 1199 (46.8) | 560 (21.8) | 2563 |
| High | 1134 (27.5) | 2152 (52.2) | 839 (20.3) | 4125 |
| Undefined or missing | 83 (51.6) | 48 (29.8) | 30 (18.6) | 161 |
P for difference between treatment groups for all variables was less than .001, the χ 2 test was used for categorical variables and the t test for continuous variables. All statistical tests are two-sided. PSA = prostate-specific antigen; WHO = World Health Organization.
In 1997, five of the six regions in Sweden participated (South, South-East, Western, Uppsala–Örebro, and Northern regions). From 1998 through 2002, all six regions in Sweden, including Stockholm–Gotland, participated in the NPCR Follow-up Study.
The low-risk category was defined as tumor stage of T1a, b, or c; Gleason score 2-6 or WHO grade of I-II; and a serum PSA level of less than 10 ng/mL. The intermediate-risk category was defined as a Gleason score of 7 or a tumor stage of T2 or a serum PSA level of 10 ng/mL or higher.
Recruitment period, age at diagnosis, tumor characteristics, comorbidity, and socioeconomic index for 6849 patients with prostate cancer: the National Prostate Cancer Register (NPCR) of Sweden Follow-up Study *
| Characteristic | Surveillance group (n = 2021) | Radical prostatectomy group (n = 3399) | Radiation therapy group (n = 1429) | Total cohort |
| Year of recruitment † , No. (%) | ||||
| 1997–1998 | 569 (41.4) | 584 (42.5) | 221 (16.1) | 1374 |
| 1999–2000 | 667 (28.6) | 1148 (49.3) | 515 (22.1) | 2330 |
| 2000–2001 | 785 (25.0) | 1667 (53.0) | 693 (22.0) | 3145 |
| Age at diagnosis | ||||
| Age group, No. (%) | ||||
| <60 y | 299 (16.3) | 1224 (66.7) | 313 (17.0) | 1836 |
| 60–64 y | 519 (25.5) | 1098 (53.9) | 419 (20.6) | 2036 |
| 65–70 y | 1203 (40.4) | 1077 (36.2) | 697 (23.4) | 2977 |
| Mean, y (SD) | 64.7 (4.6) | 61.2 (5.3) | 63.4 (4.9) | |
| Clinical local T stage, No. (%) | ||||
| T1a | 331 (83.0) | 51 (12.8) | 17 (4.3) | 399 |
| T1b | 91 (54.8) | 46 (27.7) | 29 (17.5) | 166 |
| T1c | 1021 (29.6) | 1805 (52.3) | 624 (18.1) | 3450 |
| T2 | 578 (20.4) | 1497 (52.8) | 759 (26.8) | 2834 |
| Tumor differentiation, No. (%) | ||||
| Gleason score 2–6 or WHO I or II | 1928 (32.8) | 2798 (47.6) | 1149 (19.6) | 5875 |
| Gleason score 7 | 93 (9.5) | 601 (61.7) | 280 (28.7) | 974 |
| Serum PSA level | ||||
| Category, No. (%) | ||||
| 0–4 ng/mL | 422 (48.8) | 343 (39.7) | 100 (11.6) | 865 |
| 4–10 ng/mL | 1049 (26.9) | 2064 (52.9) | 787 (20.2) | 3900 |
| 10–20 ng/mL | 550 (26.4) | 992 (47.6) | 542 (26.0) | 2084 |
| Mean, ng/mL (SD) | 7.6 (4.4) | 8.2 (3.9) | 9.3 (4.2) | |
| Risk category ‡ , No. (%) | ||||
| Low | 1085 (40.4) | 1227 (45.7) | 374 (13.9) | 2686 |
| Intermediate | 936 (22.5) | 2172 (52.2) | 1055 (25.3) | 4163 |
| Charlson comorbidity index, No. (%) | ||||
| 0–1 | 1801 (28.4) | 3233 (50.9) | 1313 (20.7) | 6347 |
| ≥2 | 220 (43.8) | 166 (33.1) | 116 (23.1) | 502 |
| Socioeconomic index, No. (%) | ||||
| Low | 804 (31.4) | 1199 (46.8) | 560 (21.8) | 2563 |
| High | 1134 (27.5) | 2152 (52.2) | 839 (20.3) | 4125 |
| Undefined or missing | 83 (51.6) | 48 (29.8) | 30 (18.6) | 161 |
| Characteristic | Surveillance group (n = 2021) | Radical prostatectomy group (n = 3399) | Radiation therapy group (n = 1429) | Total cohort |
| Year of recruitment † , No. (%) | ||||
| 1997–1998 | 569 (41.4) | 584 (42.5) | 221 (16.1) | 1374 |
| 1999–2000 | 667 (28.6) | 1148 (49.3) | 515 (22.1) | 2330 |
| 2000–2001 | 785 (25.0) | 1667 (53.0) | 693 (22.0) | 3145 |
| Age at diagnosis | ||||
| Age group, No. (%) | ||||
| <60 y | 299 (16.3) | 1224 (66.7) | 313 (17.0) | 1836 |
| 60–64 y | 519 (25.5) | 1098 (53.9) | 419 (20.6) | 2036 |
| 65–70 y | 1203 (40.4) | 1077 (36.2) | 697 (23.4) | 2977 |
| Mean, y (SD) | 64.7 (4.6) | 61.2 (5.3) | 63.4 (4.9) | |
| Clinical local T stage, No. (%) | ||||
| T1a | 331 (83.0) | 51 (12.8) | 17 (4.3) | 399 |
| T1b | 91 (54.8) | 46 (27.7) | 29 (17.5) | 166 |
| T1c | 1021 (29.6) | 1805 (52.3) | 624 (18.1) | 3450 |
| T2 | 578 (20.4) | 1497 (52.8) | 759 (26.8) | 2834 |
| Tumor differentiation, No. (%) | ||||
| Gleason score 2–6 or WHO I or II | 1928 (32.8) | 2798 (47.6) | 1149 (19.6) | 5875 |
| Gleason score 7 | 93 (9.5) | 601 (61.7) | 280 (28.7) | 974 |
| Serum PSA level | ||||
| Category, No. (%) | ||||
| 0–4 ng/mL | 422 (48.8) | 343 (39.7) | 100 (11.6) | 865 |
| 4–10 ng/mL | 1049 (26.9) | 2064 (52.9) | 787 (20.2) | 3900 |
| 10–20 ng/mL | 550 (26.4) | 992 (47.6) | 542 (26.0) | 2084 |
| Mean, ng/mL (SD) | 7.6 (4.4) | 8.2 (3.9) | 9.3 (4.2) | |
| Risk category ‡ , No. (%) | ||||
| Low | 1085 (40.4) | 1227 (45.7) | 374 (13.9) | 2686 |
| Intermediate | 936 (22.5) | 2172 (52.2) | 1055 (25.3) | 4163 |
| Charlson comorbidity index, No. (%) | ||||
| 0–1 | 1801 (28.4) | 3233 (50.9) | 1313 (20.7) | 6347 |
| ≥2 | 220 (43.8) | 166 (33.1) | 116 (23.1) | 502 |
| Socioeconomic index, No. (%) | ||||
| Low | 804 (31.4) | 1199 (46.8) | 560 (21.8) | 2563 |
| High | 1134 (27.5) | 2152 (52.2) | 839 (20.3) | 4125 |
| Undefined or missing | 83 (51.6) | 48 (29.8) | 30 (18.6) | 161 |
P for difference between treatment groups for all variables was less than .001, the χ 2 test was used for categorical variables and the t test for continuous variables. All statistical tests are two-sided. PSA = prostate-specific antigen; WHO = World Health Organization.
In 1997, five of the six regions in Sweden participated (South, South-East, Western, Uppsala–Örebro, and Northern regions). From 1998 through 2002, all six regions in Sweden, including Stockholm–Gotland, participated in the NPCR Follow-up Study.
The low-risk category was defined as tumor stage of T1a, b, or c; Gleason score 2-6 or WHO grade of I-II; and a serum PSA level of less than 10 ng/mL. The intermediate-risk category was defined as a Gleason score of 7 or a tumor stage of T2 or a serum PSA level of 10 ng/mL or higher.
Death From Competing and All Causes
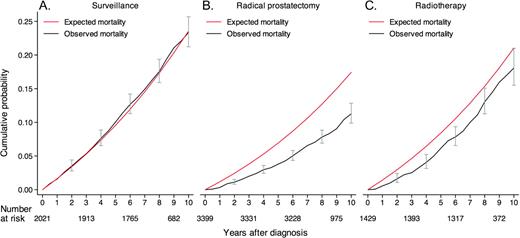
The median follow-up time was 8.2 years (interquartile range = 7.1–9.7 years), and the numbers of patients who died during follow-up were 413 (20.4%) of the 2021 patients in the surveillance group, 286 (8.4%) of the 3399 patients in the radical prostatectomy group, and 196 (13.7%) of the 1429 patients in the radiation therapy group ( Table 2 ). The observed cumulated all-cause mortality for all treatment groups combined was lower than expected (ie, in comparison with an age-matched background population). All-cause mortality in the surveillance group was similar to that of the background population, whereas all-cause mortality was lower than expected in the radiation therapy group and especially in the prostatectomy group ( Figure 2 ). The 10-year cumulative risk of dying of competing causes differed statistically significantly by treatment received and was 19.2% (95% CI = 17.2% to 21.3%) in the surveillance group, 10.2% (95% CI = 9.0% to 11.4%) in the curative intent group, including 8.5% (95% CI = 7.3% to 9.8%) in the prostatectomy group, and 14.2% (95% CI = 11.7% to 16.9%) in the radiation therapy group. These differences remained statistically significant after adjustment for age, risk category, socioeconomic status, and Charlson index ( Table 3 ). In multivariable analyses including age, risk group, treatment, socioeconomic status, and Charlson index, death from competing causes was lower among patients with higher socioeconomic status than among patients with lower socioeconomic status (RR = 0.77, 95% CI = 0.66 to 0.90), and risk of death from competing causes was higher among patients with a Charlson index of 2 or higher than among patients with Charlson index of 0–1 (RR = 3.05, 95% CI = 2.51 to 3.72).
Time at risk and cause of death according to risk category and treatment group among 6849 patients with prostate cancer in the National Prostate Cancer Register (NPCR) of Sweden Follow-up Study
| Time at risk, cause of death, and risk category | Surveillance group (n = 2021) | Radical prostatectomy group (n = 3399) | Radiation therapy group (n = 1429) |
| Man-years at risk | 15 970 | 27 118 | 11 173 |
| No. of deaths from all causes (%) | 413 (20.4) | 286 (8.4) | 196 (13.7) |
| No. of deaths from competing causes * (%) | 355 (17.6) | 230 (6.8) | 156 (10.9) |
| No. of prostate cancer–specific deaths † (%) | 58 (2.9) | 56 (1.7) | 40 (2.8) |
| Low-risk category | 14 (1.3) | 4 (0.3) | 5 (1.3) |
| Intermediate-risk category | 44 (4.7) | 52 (2.4) | 35 (3.3) |
| Time at risk, cause of death, and risk category | Surveillance group (n = 2021) | Radical prostatectomy group (n = 3399) | Radiation therapy group (n = 1429) |
| Man-years at risk | 15 970 | 27 118 | 11 173 |
| No. of deaths from all causes (%) | 413 (20.4) | 286 (8.4) | 196 (13.7) |
| No. of deaths from competing causes * (%) | 355 (17.6) | 230 (6.8) | 156 (10.9) |
| No. of prostate cancer–specific deaths † (%) | 58 (2.9) | 56 (1.7) | 40 (2.8) |
| Low-risk category | 14 (1.3) | 4 (0.3) | 5 (1.3) |
| Intermediate-risk category | 44 (4.7) | 52 (2.4) | 35 (3.3) |
Cause of death was missing for 27 patients.
The low-risk category was defined as tumor stage of T1a, b, or c; Gleason score 2-6 or World Health Organization grade of I-II; and serum prostate-specific antigen (PSA) level of less than 10 ng/mL. The intermediate-risk category was defined as Gleason score of 7 or tumor stage T2 or serum PSA level of 10 ng/mL or higher.
Time at risk and cause of death according to risk category and treatment group among 6849 patients with prostate cancer in the National Prostate Cancer Register (NPCR) of Sweden Follow-up Study
| Time at risk, cause of death, and risk category | Surveillance group (n = 2021) | Radical prostatectomy group (n = 3399) | Radiation therapy group (n = 1429) |
| Man-years at risk | 15 970 | 27 118 | 11 173 |
| No. of deaths from all causes (%) | 413 (20.4) | 286 (8.4) | 196 (13.7) |
| No. of deaths from competing causes * (%) | 355 (17.6) | 230 (6.8) | 156 (10.9) |
| No. of prostate cancer–specific deaths † (%) | 58 (2.9) | 56 (1.7) | 40 (2.8) |
| Low-risk category | 14 (1.3) | 4 (0.3) | 5 (1.3) |
| Intermediate-risk category | 44 (4.7) | 52 (2.4) | 35 (3.3) |
| Time at risk, cause of death, and risk category | Surveillance group (n = 2021) | Radical prostatectomy group (n = 3399) | Radiation therapy group (n = 1429) |
| Man-years at risk | 15 970 | 27 118 | 11 173 |
| No. of deaths from all causes (%) | 413 (20.4) | 286 (8.4) | 196 (13.7) |
| No. of deaths from competing causes * (%) | 355 (17.6) | 230 (6.8) | 156 (10.9) |
| No. of prostate cancer–specific deaths † (%) | 58 (2.9) | 56 (1.7) | 40 (2.8) |
| Low-risk category | 14 (1.3) | 4 (0.3) | 5 (1.3) |
| Intermediate-risk category | 44 (4.7) | 52 (2.4) | 35 (3.3) |
Cause of death was missing for 27 patients.
The low-risk category was defined as tumor stage of T1a, b, or c; Gleason score 2-6 or World Health Organization grade of I-II; and serum prostate-specific antigen (PSA) level of less than 10 ng/mL. The intermediate-risk category was defined as Gleason score of 7 or tumor stage T2 or serum PSA level of 10 ng/mL or higher.
Calculated cumulative prostate cancer–specific, competing, and all-cause mortality after 10 years of follow-up and difference in absolute risk and relative risk (RR) of death from prostate cancer, competing causes, and all causes according to treatment among 6849 patients with prostate cancer in the National Prostate Cancer Register (NPCR) of Sweden Follow-up Study *
| Cause of death and risk category | Cumulative mortality, % (95% CI) | Absolute risk difference, % (95% CI) | RR (95% CI) | Adjusted RR (95% CI) † | |||
| Surveillance | RP | RT | Comparison | ||||
| Prostate cancer | |||||||
| Both risk categories | 3.6 (2.7 to 4.8) | 2.4 (1.8 to 3.3) | 3.3 (2.5 to 5.7) | ||||
| RP vs Surv | 1.2 (−0.1 to 2.5) | 0.61 (0.42 to 0.88) | 0.49 (0.34 to 0.71) | ||||
| RT vs Surv | 0.3 (−1.1 to 2.9) | 1.04 (0.70 to 1.56) | 0.70 (0.45 to 1.09) | ||||
| RP vs RT | 0.9 (−1.6 to 2.1) | 0.58 (0.39 to 0.87) | 0.72 (0.47 to 1.11) | ||||
| Low-risk category ‡ | 2.4 (1.2 to 4.1) | 0.4 (0.13 to 0.97) | 1.8 (0.65 to 4.0) | ||||
| RP vs Surv | 2.0 (0.3 to 3.3) | 0.28 (0.09 to 0.83) | 0.29 (0.09 to 0.87) | ||||
| RT vs Surv | 0.6 (−1.5 to 3.1) | 1.12 (0.41 to 3.08) | 0.94 (0.31 to 2.85) | ||||
| RP vs RT | 1.4 (−0.8 to 2.7) | 0.25 (0.07 to 0.93) | 0.35 (0.08 to 1.21) | ||||
| Intermediate-risk category ‡ | 5.2 (3.7 to 6.9) | 3.4 (2.5 to 4.7) | 3.8 (2.6 to 5.4) | ||||
| RP vs Surv | 1.8 (−0.1 to 3.8) | 0.55 (0.36 to 0.82) | 0.53 (0.35 to 0.80) | ||||
| RT vs Surv | 1.4 (−0.7 to 3.6) | 0.76 (0.49 to 1.20) | 0.66 (0.42 to 1.06) | ||||
| RP vs RT | 0.4 (−1.4 to 2.2) | 0.70 (0.46 to 1.08) | 0.79 (0.51 to 1.23) | ||||
| Competing causes | |||||||
| Both risk categories | 19.2 (17.2 to 21.3) | 8.5 (7.3 to 9.8) | 14.2 (11.7 to 16.9) | ||||
| RP vs Surv | 10.7 (8.3 to 13.1) | 0.39 (0.33 to 0.46) | 0.48 (0.40 to 0.58) | ||||
| RT vs Surv | 5.0 (1.7 to 8.4) | 0.64 (0.52 to 0.77) | 0.67 (0.55 to 0.83) | ||||
| RP vs RT | 5.7 (2.7 to 8.5) | 0.61 (0.50 to 0.75) | 0.72 (0.58 to 0.89) | ||||
| All causes | |||||||
| Both risk categories | 23.4 (21.3 to 25.8) | 11.3 (10.0 to 12.9) | 18.3 (15.7 to 21.3) | ||||
| RP vs Surv | 12.1 (9.4 to 14.7) | 0.42 (0.36 to 0.48) | 0.49 (0.41 to 0.57) | ||||
| RT vs Surv | 5.1 (1.6 to 8.8) | 0.70 (0.59 to 0.83) | 0.68 (0.57 to 0.82) | ||||
| RP vs RT | 7.0 (3.7 to 10.1) | 0.60 (0.50 to 0.71) | 0.71 (0.59 to 0.85) | ||||
| Cause of death and risk category | Cumulative mortality, % (95% CI) | Absolute risk difference, % (95% CI) | RR (95% CI) | Adjusted RR (95% CI) † | |||
| Surveillance | RP | RT | Comparison | ||||
| Prostate cancer | |||||||
| Both risk categories | 3.6 (2.7 to 4.8) | 2.4 (1.8 to 3.3) | 3.3 (2.5 to 5.7) | ||||
| RP vs Surv | 1.2 (−0.1 to 2.5) | 0.61 (0.42 to 0.88) | 0.49 (0.34 to 0.71) | ||||
| RT vs Surv | 0.3 (−1.1 to 2.9) | 1.04 (0.70 to 1.56) | 0.70 (0.45 to 1.09) | ||||
| RP vs RT | 0.9 (−1.6 to 2.1) | 0.58 (0.39 to 0.87) | 0.72 (0.47 to 1.11) | ||||
| Low-risk category ‡ | 2.4 (1.2 to 4.1) | 0.4 (0.13 to 0.97) | 1.8 (0.65 to 4.0) | ||||
| RP vs Surv | 2.0 (0.3 to 3.3) | 0.28 (0.09 to 0.83) | 0.29 (0.09 to 0.87) | ||||
| RT vs Surv | 0.6 (−1.5 to 3.1) | 1.12 (0.41 to 3.08) | 0.94 (0.31 to 2.85) | ||||
| RP vs RT | 1.4 (−0.8 to 2.7) | 0.25 (0.07 to 0.93) | 0.35 (0.08 to 1.21) | ||||
| Intermediate-risk category ‡ | 5.2 (3.7 to 6.9) | 3.4 (2.5 to 4.7) | 3.8 (2.6 to 5.4) | ||||
| RP vs Surv | 1.8 (−0.1 to 3.8) | 0.55 (0.36 to 0.82) | 0.53 (0.35 to 0.80) | ||||
| RT vs Surv | 1.4 (−0.7 to 3.6) | 0.76 (0.49 to 1.20) | 0.66 (0.42 to 1.06) | ||||
| RP vs RT | 0.4 (−1.4 to 2.2) | 0.70 (0.46 to 1.08) | 0.79 (0.51 to 1.23) | ||||
| Competing causes | |||||||
| Both risk categories | 19.2 (17.2 to 21.3) | 8.5 (7.3 to 9.8) | 14.2 (11.7 to 16.9) | ||||
| RP vs Surv | 10.7 (8.3 to 13.1) | 0.39 (0.33 to 0.46) | 0.48 (0.40 to 0.58) | ||||
| RT vs Surv | 5.0 (1.7 to 8.4) | 0.64 (0.52 to 0.77) | 0.67 (0.55 to 0.83) | ||||
| RP vs RT | 5.7 (2.7 to 8.5) | 0.61 (0.50 to 0.75) | 0.72 (0.58 to 0.89) | ||||
| All causes | |||||||
| Both risk categories | 23.4 (21.3 to 25.8) | 11.3 (10.0 to 12.9) | 18.3 (15.7 to 21.3) | ||||
| RP vs Surv | 12.1 (9.4 to 14.7) | 0.42 (0.36 to 0.48) | 0.49 (0.41 to 0.57) | ||||
| RT vs Surv | 5.1 (1.6 to 8.8) | 0.70 (0.59 to 0.83) | 0.68 (0.57 to 0.82) | ||||
| RP vs RT | 7.0 (3.7 to 10.1) | 0.60 (0.50 to 0.71) | 0.71 (0.59 to 0.85) | ||||
CI = confidence interval; RP = radical prostatectomy group; RT = radiation therapy group; Surv = surveillance group.
This analysis was adjusted for comorbidity, socioeconomic group, risk group (when relevant), and age at diagnosis.
The low-risk category was defined as tumor stage T1a, b, or c; Gleason score 2-6 or World Health Organization grade I-II; and serum prostate-specific antigen (PSA) level of less than 10 ng/mL. The intermediate-risk category was defined as a Gleason score of 7 or tumor stage T2 or serum PSA level of 10 ng/mL or higher.
Calculated cumulative prostate cancer–specific, competing, and all-cause mortality after 10 years of follow-up and difference in absolute risk and relative risk (RR) of death from prostate cancer, competing causes, and all causes according to treatment among 6849 patients with prostate cancer in the National Prostate Cancer Register (NPCR) of Sweden Follow-up Study *
| Cause of death and risk category | Cumulative mortality, % (95% CI) | Absolute risk difference, % (95% CI) | RR (95% CI) | Adjusted RR (95% CI) † | |||
| Surveillance | RP | RT | Comparison | ||||
| Prostate cancer | |||||||
| Both risk categories | 3.6 (2.7 to 4.8) | 2.4 (1.8 to 3.3) | 3.3 (2.5 to 5.7) | ||||
| RP vs Surv | 1.2 (−0.1 to 2.5) | 0.61 (0.42 to 0.88) | 0.49 (0.34 to 0.71) | ||||
| RT vs Surv | 0.3 (−1.1 to 2.9) | 1.04 (0.70 to 1.56) | 0.70 (0.45 to 1.09) | ||||
| RP vs RT | 0.9 (−1.6 to 2.1) | 0.58 (0.39 to 0.87) | 0.72 (0.47 to 1.11) | ||||
| Low-risk category ‡ | 2.4 (1.2 to 4.1) | 0.4 (0.13 to 0.97) | 1.8 (0.65 to 4.0) | ||||
| RP vs Surv | 2.0 (0.3 to 3.3) | 0.28 (0.09 to 0.83) | 0.29 (0.09 to 0.87) | ||||
| RT vs Surv | 0.6 (−1.5 to 3.1) | 1.12 (0.41 to 3.08) | 0.94 (0.31 to 2.85) | ||||
| RP vs RT | 1.4 (−0.8 to 2.7) | 0.25 (0.07 to 0.93) | 0.35 (0.08 to 1.21) | ||||
| Intermediate-risk category ‡ | 5.2 (3.7 to 6.9) | 3.4 (2.5 to 4.7) | 3.8 (2.6 to 5.4) | ||||
| RP vs Surv | 1.8 (−0.1 to 3.8) | 0.55 (0.36 to 0.82) | 0.53 (0.35 to 0.80) | ||||
| RT vs Surv | 1.4 (−0.7 to 3.6) | 0.76 (0.49 to 1.20) | 0.66 (0.42 to 1.06) | ||||
| RP vs RT | 0.4 (−1.4 to 2.2) | 0.70 (0.46 to 1.08) | 0.79 (0.51 to 1.23) | ||||
| Competing causes | |||||||
| Both risk categories | 19.2 (17.2 to 21.3) | 8.5 (7.3 to 9.8) | 14.2 (11.7 to 16.9) | ||||
| RP vs Surv | 10.7 (8.3 to 13.1) | 0.39 (0.33 to 0.46) | 0.48 (0.40 to 0.58) | ||||
| RT vs Surv | 5.0 (1.7 to 8.4) | 0.64 (0.52 to 0.77) | 0.67 (0.55 to 0.83) | ||||
| RP vs RT | 5.7 (2.7 to 8.5) | 0.61 (0.50 to 0.75) | 0.72 (0.58 to 0.89) | ||||
| All causes | |||||||
| Both risk categories | 23.4 (21.3 to 25.8) | 11.3 (10.0 to 12.9) | 18.3 (15.7 to 21.3) | ||||
| RP vs Surv | 12.1 (9.4 to 14.7) | 0.42 (0.36 to 0.48) | 0.49 (0.41 to 0.57) | ||||
| RT vs Surv | 5.1 (1.6 to 8.8) | 0.70 (0.59 to 0.83) | 0.68 (0.57 to 0.82) | ||||
| RP vs RT | 7.0 (3.7 to 10.1) | 0.60 (0.50 to 0.71) | 0.71 (0.59 to 0.85) | ||||
| Cause of death and risk category | Cumulative mortality, % (95% CI) | Absolute risk difference, % (95% CI) | RR (95% CI) | Adjusted RR (95% CI) † | |||
| Surveillance | RP | RT | Comparison | ||||
| Prostate cancer | |||||||
| Both risk categories | 3.6 (2.7 to 4.8) | 2.4 (1.8 to 3.3) | 3.3 (2.5 to 5.7) | ||||
| RP vs Surv | 1.2 (−0.1 to 2.5) | 0.61 (0.42 to 0.88) | 0.49 (0.34 to 0.71) | ||||
| RT vs Surv | 0.3 (−1.1 to 2.9) | 1.04 (0.70 to 1.56) | 0.70 (0.45 to 1.09) | ||||
| RP vs RT | 0.9 (−1.6 to 2.1) | 0.58 (0.39 to 0.87) | 0.72 (0.47 to 1.11) | ||||
| Low-risk category ‡ | 2.4 (1.2 to 4.1) | 0.4 (0.13 to 0.97) | 1.8 (0.65 to 4.0) | ||||
| RP vs Surv | 2.0 (0.3 to 3.3) | 0.28 (0.09 to 0.83) | 0.29 (0.09 to 0.87) | ||||
| RT vs Surv | 0.6 (−1.5 to 3.1) | 1.12 (0.41 to 3.08) | 0.94 (0.31 to 2.85) | ||||
| RP vs RT | 1.4 (−0.8 to 2.7) | 0.25 (0.07 to 0.93) | 0.35 (0.08 to 1.21) | ||||
| Intermediate-risk category ‡ | 5.2 (3.7 to 6.9) | 3.4 (2.5 to 4.7) | 3.8 (2.6 to 5.4) | ||||
| RP vs Surv | 1.8 (−0.1 to 3.8) | 0.55 (0.36 to 0.82) | 0.53 (0.35 to 0.80) | ||||
| RT vs Surv | 1.4 (−0.7 to 3.6) | 0.76 (0.49 to 1.20) | 0.66 (0.42 to 1.06) | ||||
| RP vs RT | 0.4 (−1.4 to 2.2) | 0.70 (0.46 to 1.08) | 0.79 (0.51 to 1.23) | ||||
| Competing causes | |||||||
| Both risk categories | 19.2 (17.2 to 21.3) | 8.5 (7.3 to 9.8) | 14.2 (11.7 to 16.9) | ||||
| RP vs Surv | 10.7 (8.3 to 13.1) | 0.39 (0.33 to 0.46) | 0.48 (0.40 to 0.58) | ||||
| RT vs Surv | 5.0 (1.7 to 8.4) | 0.64 (0.52 to 0.77) | 0.67 (0.55 to 0.83) | ||||
| RP vs RT | 5.7 (2.7 to 8.5) | 0.61 (0.50 to 0.75) | 0.72 (0.58 to 0.89) | ||||
| All causes | |||||||
| Both risk categories | 23.4 (21.3 to 25.8) | 11.3 (10.0 to 12.9) | 18.3 (15.7 to 21.3) | ||||
| RP vs Surv | 12.1 (9.4 to 14.7) | 0.42 (0.36 to 0.48) | 0.49 (0.41 to 0.57) | ||||
| RT vs Surv | 5.1 (1.6 to 8.8) | 0.70 (0.59 to 0.83) | 0.68 (0.57 to 0.82) | ||||
| RP vs RT | 7.0 (3.7 to 10.1) | 0.60 (0.50 to 0.71) | 0.71 (0.59 to 0.85) | ||||
CI = confidence interval; RP = radical prostatectomy group; RT = radiation therapy group; Surv = surveillance group.
This analysis was adjusted for comorbidity, socioeconomic group, risk group (when relevant), and age at diagnosis.
The low-risk category was defined as tumor stage T1a, b, or c; Gleason score 2-6 or World Health Organization grade I-II; and serum prostate-specific antigen (PSA) level of less than 10 ng/mL. The intermediate-risk category was defined as a Gleason score of 7 or tumor stage T2 or serum PSA level of 10 ng/mL or higher.
Observed and expected all-cause mortality for patients in the National Prostate Cancer Register (NPCR) of Sweden Follow-up Study. A ) Patients who were treated with surveillance. B ) Patients who were treated with radical prostatectomy. C ) Patients who were treated with radiation therapy. Error bars = 95% confidence intervals.
Prostate Cancer–Specific Mortality
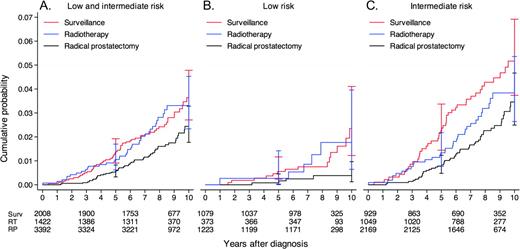
Death was attributed to prostate cancer in 58 (2.9%) of the 2021 patients in the surveillance group, in 56 (1.7%) of the 3339 patients in the prostatectomy group, and in 40 (2.8%) of the 1429 patients in the radiation therapy group ( Table 2 ). The calculated cumulated prostate cancer–specific mortality after 10 years of follow-up was 3.6% (95% CI = 2.7% to 4.8%) in the surveillance group and 2.7% (95% CI = 2.1% to 3.4%) in the curative intent groups, including the prostatectomy group (2.4%, 95% CI = 1.8% to 3.3%) and the radiation therapy group (3.3%, 95% CI = 2.5% to 5.7%) ( Table 3 and Figure 3 ). Among those with low-risk disease, prostate cancer–specific mortality was 2.4% (95% CI = 1.2% to 4.1%) in the surveillance group and 0.7% (95% CI = 0.3% to 1.4%) in the curative intent groups, including the prostatectomy group (0.4%, 95% CI = 0.13% to 0.97%) and the radiation therapy group (1.8%, 95% CI = 0.65% to 4.0%). Among those in the intermediate-risk category, prostate cancer–specific mortality was more than twice as high as for those in the low-risk category: 5.2% (95% CI = 3.7% to 6.9%) in the surveillance group, 3.4% (95% CI = 2.5% to 4.7%) in the prostatectomy group, and 3.8% (95% CI = 2.6% to 5.4%) in the radiation therapy group.
Prostate cancer–specific mortality for patients who were treated with surveillance, radiation therapy, or radical prostatectomy in the National Prostate Cancer Register (NPCR) of Sweden Follow-up Study. A ) Combination of low- and intermediate-risk patients. B ) Low-risk patients. C ) Intermediate-risk patients. Error bars = 95% confidence intervals. RP = radical prostatectomy; RT = radiation therapy; Surv = surveillance.
After adjustment for risk category, Charlson index, and socioeconomic status, there was a lower risk of prostate cancer–specific mortality among those in the prostatectomy group than among those in the surveillance group (RR = 0.49, 95% CI = 0.34 to 0.71), among those in the radiation therapy group than among those in the surveillance group (RR = 0.70, 95% CI = 0.45 to 1.09), and among those in the prostatectomy group than among those in the radiation therapy group (RR = 0.72, 95% CI = 0.47 to 1.11). In a multivariable analysis, prostate cancer–specific mortality was lower among patients with higher socioeconomic status than those with lower socioeconomic status (RR = 0.69, 95% CI = 0.50 to 0.95), and prostate cancer–specific mortality was also lower among those with high comorbidity (for those with a Charlson index of ≥2 compared with those with a Charlson index of 0–1, RR = 0.24, 95% CI = 0.08 to 0.75).
Discussion
In this nationwide population-based cohort of 6849 patients with low- or intermediate-risk prostate cancer in Sweden, risk of prostate cancer–specific death was low at up to 8.2 years after diagnosis regardless of treatment strategy, with only 154 (17%) of the 895 deaths being attributed to prostate cancer. Cumulative 10-year prostate cancer–specific mortality was 3.6% for patients in the surveillance group and 2.7% for those in the curative intent groups. Among the group of 2686 patients with low-risk prostate cancer, the corresponding prostate cancer–specific mortality was 2.4% for patients in the surveillance group and 0.7% for those in the curative intent groups.
Death From Competing Causes and All Causes, Confounding by Indication and Selection Bias
All-cause mortality in the full cohort was lower than expected, indicating that, in clinical practice, there is a selection of healthy men for PSA testing and further work-up, leading to a diagnosis of localized prostate cancer. There was a much higher percentage of death from competing causes among patients in the surveillance group (17.6%) than among patients in the prostatectomy group (6.8%) or in the radiation therapy group (10.9%). Thus, patients with a short life expectancy were more often selected for surveillance than for surgery or radiation therapy. We tried to adjust for this selection bias by including into analysis indices indicative of socioeconomic status and comorbidity, which are known to be associated with life expectancy. However, when we adjusted the model for comorbidity and socioeconomic status, risk estimates of death from competing and all causes were not materially different from unadjusted estimates. Thus, adjustments for these indices were not sufficient to compensate for the confounding by indication to treatment caused by treatment selection in clinical patient management in our cohort. Giordano et al. ( 32 ) similarly tried to compensate for confounding in a study of localized prostate cancer that used data in SEER and Medicare files, but also they found that a large difference in all-cause mortality, mainly from competing causes of death, still remained after adjustments for year of diagnosis, age, race, urban residence, marital status, income, education, SEER region, tumor size, tumor grade, and comorbidity score. Both the study by Giordano et al. and the NPCR Follow-up Study show that adjustment for comorbidity and socioeconomic status by use of register-derived data cannot compensate for the bias and confounding that occurs in observational studies that are based on routine clinical management. Thus, all-cause mortality is not a valid endpoint in evaluating treatment effects in observational studies of localized prostate cancer because most patients with localized prostate cancer die from competing causes and because the confounding is strongly related to competing causes of death, relative survival is likewise not a useful measure.
Prostate Cancer–Specific Mortality Among Patients on Surveillance and Patients on Curative Treatment
Fewer patients with intermediate-risk tumors received surveillance (n = 936) than curative treatment (n = 3227), and other adverse tumor factors that were not possible to study in our analyses (eg, tumor extent on biopsy examination) also appear to have been less adverse among those on surveillance. Thus, there was also confounding by indication for tumor-related factors, but tumor factors had a weaker effect on all-cause mortality than factors related to overall health. Furthermore, 484 (24%) of the 2021 patients in the surveillance group subsequently received curative treatment during follow-up. Despite these biases in overall health, tumor-related factors, and initiation of deferred treatment that would decrease the difference in prostate cancer–specific mortality between those in the surveillance group and those in the curative treatment group, the risk of calculated cumulative prostate cancer–specific death was statistically significantly lower among patients in the prostatectomy group than among patients in the surveillance group (RR = 0.49, 95% CI = 0.34 to 0.71), but the difference in absolute risk between the groups was still very modest (1.2%) after 10 years of follow-up. The difference is in the same direction and in same order of magnitude as that observed in the randomized SPCG-4 trial (RR = 0.65, 95% CI = 0.45 to 0.94) ( 11 ); however, we acknowledge that the NPCR Follow-up Study was not well suited to quantify the difference in effect of various treatment strategies on prostate cancer–specific mortality because of confounding that was related to overall health and prostate cancer aggressiveness.
Comparison with Published Observational Studies on Surveillance
Some single-institution studies ( 6–9 ) of active surveillance for localized prostate cancer have reported favorable outcome. The inclusion criteria for these series have generally included well-differentiated tumors as reflected by a Gleason score of 6 or lower, clinical local stage of T2 or lower, serum PSA levels of less than 10 ng/mL, PSA density of less than 0.2 ng/mL per cm 3 , tumor extent on core biopsy examination that was limited to a maximum of two cores involved, and follow-up with PSA testing every 3–6 months and re-biopsy examination at 1–2 years after diagnosis. These studies ( 6–9 ) have also reported low progression rates, especially, Klotz ( 6 , 8 ) observed a prostate cancer–specific mortality of 1% among 299 patients with low-risk prostate cancer who had been followed for a median time of 7 years compared with the 10-year prostate cancer–specific mortality of 2.4% among 1085 patients with low-risk tumors (ie, Gleason score 2-6, T1 tumors, and serum PSA level of <10 ng/mL) in the NPCR Follow-up Study. Many of the men on surveillance in the NPCR Follow-up cohort would not have fulfilled the inclusion criteria in contemporary surveillance protocols and were followed less rigorously. Thus, we expect that prostate cancer–specific mortality among patients with low-risk prostate cancer on contemporary active surveillance protocols will be lower than such mortality that we reported in this study.
In contrast to the NPCR Follow-up Study and the observational studies ( 6–9 ) of surveillance that included patients with low-risk tumors and favorable outcome, which mirrors the current trend toward earlier stage at diagnosis ( 3 ), tumor characteristics were much more adverse in the randomized SPCG-4 trial ( 11 ) in 1989–1999 (ie, approximately 10% of the patients had stage T1c tumors) than those in the NPCR Follow-up cohort (ie, 50% of patients had stage T1c tumors). Prostate cancer–specific mortality was five times higher in SPCG-4 than in the NPCR Follow-up Study. For example, the absolute risk of prostate cancer death in the prostatectomy group was 12.5% in the SPCG-4 trial compared with 2.4% in the NPCR Follow-up cohort, and it was 17.9% in the SPCG-4 control group compared with 3.6% in the surveillance group in the NPCR cohort.
In the recent SEER study ( 12 ) on outcomes after conservative treatment of elderly US patients diagnosed with prostate cancer in 1992–2002 who had a mean age at diagnosis of 78 years and of whom 40% had received primary androgen deprivation therapy, the 10-year prostate cancer–specific mortality was 8% among the 222 patients with well-differentiated tumors (Gleason scores 2–4) and 9% among the 1088 patients with moderately differentiated tumors (Gleason scores 5–7) with unknown serum PSA levels. In total, 60% of these elderly patients died during follow-up, showing that overall health as well as age were quite different from currently diagnosed prostate cancer patients contemplating active surveillance.
Two systematic reviews ( 33 , 34 ) on the outcome after external beam radiation therapy in patients with localized prostate cancer reported a wide range of outcomes. Low mortality rates have been reported after high-dose radiation therapy ( 35 ). However, in the NPCR Follow-up cohort, we did not have detailed data on the radiation doses, a higher proportion of patients who received radiation therapy had intermediate-risk tumors than patients on surveillance or who received surgery, and prostate cancer–specific mortality was not statistically significantly lower in patients who received radiation therapy than in patients on surveillance.
This study had several strengths. The NPCR Follow-up Study was population based and included men from all types of medical facilities in the entire nation of Sweden. On the basis of a 98% capture rate of the Swedish Cancer Register of all prostate cancer patient data, a 98% capture rate for the NPCR of Sweden vs the Cancer Register, and a 94% capture rate for the Follow-up Study, we included 90% of all patients in Sweden aged 70 years or younger with a low- or intermediate-risk prostate cancer who were diagnosed from January 1, 1997, through December 31, 2002. We had data on tumor stage, differentiation, and serum PSA level and primary treatment from NPCR. These data were verified, and termination of surveillance and initiation of deferred treatment was assessed in a review of medical records. We also had access to data on socioeconomic status and comorbidity by linkage to other national registries. The reliability of death certificates in the Swedish Cause of Death Register for a correct assessment of cause of death among patients with prostate cancer has been shown to be rather high; 88% of deaths that had been attributed to prostate cancer among patients with localized disease were found to be attributed to prostate cancer in a reexamination of medical records ( 36 ). Thus, the results from the NPCR Follow-up Study population appear to be representative of contemporary patient outcomes in Sweden as obtained in clinical practice.
This study had several limitations. The observational design resulted in a strong selection bias in which a higher proportion of healthy patients with prostate cancer with adverse factors were assigned to radical prostatectomy than to surveillance. No information was available for the NPCR Follow-up cohort on tumor extent in core biopsy specimens, serum PSA levels after the date of diagnosis, or progression to metastatic disease. The median follow-up time was limited to 8.2 years, and because most patients currently diagnosed with localized prostate cancer are aged 60–70 years and have a life expectancy of more than 15 years, longer follow-up is needed.
In conclusion, with a 10-year prostate cancer–specific mortality of less than 3% for patients with low-risk prostate cancer on surveillance, this strategy appears to be suitable for many of these men. However, additional follow-up is required for conclusive evaluation of surveillance as treatment strategy in prostate cancer.
Funding
The Swedish Research Council (5910); the Swedish Cancer Foundation (0750); Västerbotten County Council (75171).
References
J. Hugosson and J.-E. Johansson designed the Follow-up Study; P. Stattin designed the analysis in this report and drafted and wrote the manuscript; E. Holmberg had full access to data and is responsible for data management and analysis; J. Adolfsson and L. Holmberg provided intellectual input in analysis and review of manuscript; and all authors read, approved, and take responsibility for the final manuscript. We thank Karin Hellström and the research nurses who extracted data for the Follow-up Study: Marianne Sandborg, Maria Nyberg, Ann-Sofi Isaksson, Birgitta Gobén, Ann Andersson, Eva Beckman, Kristina Grama, Inger Andersson, Ingegerd Lönn, Mia Nyman, Sylvia Dahlén, Lars Hjertzell, Agneta Grapne, Per Nanzén, Pirjo Larsson, Christina Danewid, Jeanette Ceberg, Charlotte Ingri, Siri Blomqvist, and Gerd Strandberg.
None of the authors have conflicts of interest that are relevant to the subject matter or materials discussed in the manuscript.
The funding organizations had no influence in the design and conduct of the study; collection, management, analysis, and interpretation of data; and preparation, review, or approval of the manuscript.
NPCR steering group: P. Stattin (Chair), Anders Widmark, Stefan Karlsson, Magnus Törnblom, J. Adolfsson, Anna Bill-Axelson, Ove Andrén, David Robinson, Bill Pettersson, J. Hugosson, Jan-Erik Damber, Ola Bratt, Göran Ahlgren, Lars Egevad, and Roy Ehrnström.