-
PDF
- Split View
-
Views
-
Cite
Cite
Eric Boersma, The Primary Coronary Angioplasty vs. Thrombolysis (PCAT)-2 Trialists' Collaborative Group, Does time matter? A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in-hospital fibrinolysis in acute myocardial infarction patients, European Heart Journal, Volume 27, Issue 7, April 2006, Pages 779–788, https://doi.org/10.1093/eurheartj/ehi810
Close - Share Icon Share
Abstract
Aims Although outcomes after acute myocardial infarction (AMI) seemed to be superior with primary percutaneous coronary intervention (PPCI) relative to fibrinolysis (FL), the extent to which treatment delay modulates this treatment effect is unclear.
Methods and results Twenty-five randomized trials (n=7743) testing the efficacy of PPCI vs. FL were identified in journal articles and abstract listings published between 1990 and 2002. Of these, individual patient data from 22 trials (n=6763) were pooled, and multi-level logistic regression assessed the relationship among treatment, treatment delay, and 30-day mortality. Treatment delay was divided into ‘presentation delay’ [symptom onset to randomization; FL: median 143 (IQR: 91–225) min; PPCI: 140 (91–220) min] and hospital-specific ‘PCI-related delay’ [median time from randomization to PPCI minus median time to FL per hospital; median 55 (IQR: 37–74) min]. PPCI was associated with a significant 37% reduction in 30-day mortality [adjusted OR, 0.63; 95% CI (0.42–0.84)]. Although, there was no heterogeneity in the treatment effect by presentation delay (pBreslow-Day=0.88), the absolute mortality reduction by PPCI widened over time (1.3% 0–1 h to 4.2% >6 h after symptom onset). When the PCI-related delay was <35 min, the relative (67 vs. 28% pBreslow-Day=0.004) and absolute (5.4 vs. 2.0%) mortality reduction was significantly higher than those with longer delays.
Conclusion PPCI was associated with significantly lower 30-day mortality relative to FL, regardless of treatment delay. Although logistic and economic constraints challenge the feasibility of ‘PPCI-for-all’, the benefit of timely treatment underscores the importance of a comprehensive, unified approach to delivery of cardiac care in all AMI patients.
See page 761 for the editorial comment on this article (doi:10.1093/eurheartj/ehi775)
Introduction
‘Time is myocardium’, a familiar adage in the cardiovascular community, is central to the controversy of the best modality of reperfusion after acute myocardial infarction (AMI).1,2 Although numerous pharmaceutical and mechanical treatment strategies have helped to further the quest for an absolute 1% mortality reduction after AMI, shortening the time to treatment may make this reduction attainable. Several studies, for example, have established that if fibrinolytic therapy is initiated within 3 h of symptom onset, early mortality can be reduced by 25–30% as compared with conservative therapy. If treated later, only a 15% reduction may be observed.3–5
Obtaining and maintaining arterial patency, however, remains a formidable challenge to fibrinolytic therapy.3,6,7 Whereas normal coronary flow, as measured by Thrombolysis in Myocardial Infarction (TIMI) grade 3, is restored in 29–54% of patients receiving fibrinolysis (FL), primary percutaneous coronary intervention (PPCI) is associated with normal epicardial flow in more than 90% of patients.7,8 PPCI, however, is not without limitations. Although normal coronary flow may be achieved in the epicardial arteries, flow in the distal microvascular beds may be compromised in a considerable portion of patients by microscopic atherosclerotic debris which becomes dislodged during the procedure.9,10 Similarly, mechanical reperfusion is also associated with major systemic bleeding in excess of 2% relative to pharmacologic strategies.11 The treatment delay associated with mobilizing the interventional team and arranging patient transfer to the nearest interventional facility also presents a considerable challenge to timely treatment, especially as most facilities do not operate during ‘off-hours’. Under optimal circumstances, this will lead to at least a 30 min treatment-related delay as compared with in-hospital initiation of fibrinolytic therapy.12 When compared with pre-hospital treatment with FL, this delay may range from 60 to 90 min. Outside of the clinical trial setting, fewer than 30% of PPCI patients had a door-to-balloon time less than 90 min according to findings from the National Registry of Myocardial Infarction (NRMI).13 Transfers from other institutions lengthened door-to-balloon time considerably, with less than 5% of transfer patients undergoing PPCI within 90 min after first medical contact.14
Recent evidence from several large trials suggests that the maximum benefits of either treatment strategy may occur at different time points after symptom onset. With transportation times of up to 2 h, the clinical benefits of PPCI over FL were not modified.15,16 In contrast, the PRimary Angioplasty in patients transferred from General community hospitals to specialized PTCA Units with or without Emergency thrombolysis (PRAGUE)-2 study observed no difference in 30-day mortality between PPCI and FL for patients presenting within 3 h,17 and the Comparison of Primary Angioplasty and Prehospital Thrombolysis in the Acute Phase of Myocardial Infarction (CAPTIM) trial provided evidence for a better outcome in patients receiving pre-hospital FL as compared with PPCI, provided that fibrinolytic therapy is initiated within 2 h after symptom onset.18,19
Given these recent findings, the aim of the current study was to assess whether the clinical benefit of PPCI compared with in-hospital FL is modulated by the time to treatment in a pooled analysis of randomized clinical trials reported since 1990 (Figure 1).
Methods
Trial selection
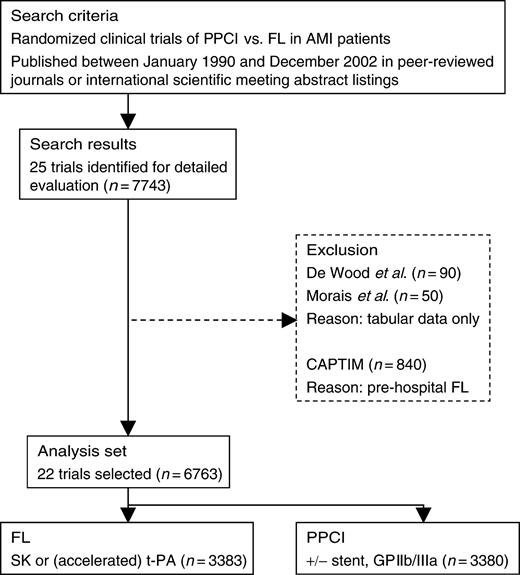
All randomized trials that enrolled at least 50 patients presenting with AMI assigned to treatment with FL or PPCI were considered. Trials published between January 1990 and December 2002 were identified by OVID MEDLINE and ISI Web of Science® using a broad range of key words, including ‘(acute) myocardial infarction’, ‘FL’, ‘fibrinolytic’, ‘thrombolysis’, ‘thrombolytic’, ‘streptokinase’, ‘tissue plasminogen activator’, ‘accelerated tissue plasminogen activator’, ‘t-PA’, ‘rt-PA’, ‘primary’, ‘angioplasty’, ‘stent’, and ‘PCI’. Non-English articles were not excluded. References of identified papers and abstract listings of annual meetings of the American Heart Association (Circulation), American College of Cardiology (Journal of the American College of Cardiology), and European Society of Cardiology (European Heart Journal) were also examined during the same period. Each trial identified in this search was critically and independently evaluated by three investigators (E Boersma, RJ Simes, and CM Westerhout) for patient population, study treatment, protocol, and endpoints. The primary investigators of these studies were contacted for verification and access to individual patient data.
Twenty-five trials, which enrolled 7743 patients, met the abovementioned search criteria (Appendix B, Table B1).15–18,20–40 Individual patient data were unavailable in two trials (DeWood et al.,27n=90; Morais et al.,32n=50) and the CAPTIM investigators judged that their protocol was incompatible with the other trials included in this pooled analysis, which excluded an additional 840 patients. Thus, individual patient data from 22 trials (n=6763) were pooled for the primary analysis. Data were assessed for completeness, internal consistency of patient records, and consistency with published reports. Any discrepancies between analysis of the data provided and previously published results were queried and resolved with the primary investigator of the trial.
Endpoint definition
The primary endpoint of this pooled analysis was all-cause mortality at 30 days. Individually, most trials were not designed (and were underpowered) to evaluate differences in mortality between the treatment strategies; however, pooling these studies provided sufficient power, particularly for subgroup analyses such as those involving time. Other endpoints (i.e. re-infarction and stroke) remained defined according to the original trial-specific criteria.
Statistical analysis
Continuous data were summarized and presented as median values with corresponding IQR, whereas dichotomous data were presented as counts and percentages. Differences in baseline characteristics between subgroups of patients were evaluated by Wilcoxon's rank sum tests, Kruskal–Wallis tests or χ2 tests as appropriate.
Time to treatment was categorized into ‘presentation delay’ (i.e. time from symptom onset to randomization) and ‘treatment delay’ (i.e. time from randomization to treatment). Patients were further categorized for presentation delays as 0–1, >1–2, >2–3, >3–6, and >6 h, which was determined a priori and based on previously published studies. Unlike presentation delay, the interval between randomization and treatment is influenced by allocated treatment, and analyses that are based on these observations after randomization in individual patients can result in biased estimates of treatment effect. Analyses based on observations at a hospital level may help to overcome this. Thus, the median time between randomization and the start of treatment (i.e. first injection of the fibrinolytic agent or the first balloon inflation) was calculated for each of the 153 hospitals. The hospital-specific difference between these median times was then determined, which is hereafter referred to as ‘PCI-related delay’, and assigned each patient within that hospital. PCI-related delay was then grouped into quintiles: 0–35, >35–50, >50–62, >62–79, and >79 min. PCI-related delay should be interpreted as the additional time that is needed to start the PCI procedure after treatment with a fibrinolytic agent could have been started.
All analyses were performed according to intention-to-treat principles. Trial-specific outcome data were pooled using the Cochrane–Mantel–Haenszel method, and OR and 95% CI for 30-day death are reported. The Breslow-Day test was applied to examine the statistical evidence of heterogeneity among the trial-specific OR. It should be noted that statistical tests for heterogeneity often lack power, and fail to elucidate differences which may be clinically important.41
Initially, the influence of presentation delay, as well as PCI-related delay, on the treatment effect at 30 days (i.e. death) was examined using single-level logistic regression. However, given the hierarchical nature of these data (i.e. patients nested within hospitals), multilevel logistic regression was then applied to address random and fixed effects at the patient and hospital levels of the study. At the patient level, age, gender, weight, diabetes mellitus, previous MI, prior revascularization [PCI or coronary artery bypass graft (CABG)], anterior MI at presentation, heart rate, systolic blood pressure, presentation delay, and study treatment (FL or PPCI) were considered fixed effects, and statistical significance was evaluated using the t-ratio.42 At the hospital level, the average annual PCI volume and PCI-related delay were available at each of the 153 hospitals. The annual PCI volume (on study only) was calculated as the average number of patients randomized to PPCI per year, which was then grouped into tertiles of its distribution (<10, 10–23, ≥24 PPCI/year). At the study level, the likelihood of PCI within 30 days after initial FL, use of stents, use of glycoprotein (GP) IIb/IIIa inhibitors, type of fibrinolytic agent used (streptokinase, t-PA, or accelerated t-PA), single-centred vs. multi-centred trial, and the year of publication were considered. When three-level models were tested, there was no variance at the study-level, which provided statistical support that these studies could be pooled for analysis. In addition to these main effects, two interactions were considered: (i) study treatment and presentation delay (patient-level interaction); and (ii) study treatment and PCI-related delay (patient/hospital-level interaction). MLwiN© (version 1.10.0007) statistical software, with residual iterated generalized least squares and pseudoquasi-likelihood model specifications, was used for the multilevel modelling.
Further analyses of the presentation delay treatment association were performed in a priori-selected patient subgroups [<65 vs. ≥65-years-old; female/male; diabetes mellitus; previous MI; anterior vs. non-anterior MI location; systolic blood pressure (<130 vs. ≥130 mmHg); heart rate (<70 vs. ≥70 bpm)], hospital-level average annual PCI volume and study-level type of fibrinolytic agent used.
Two sensitivity analyses, using single-level logistic regression were performed: (i) impact of exclusion of three trials without individual patient data;18,27,32 and (ii) type of fibrinolytic agent used (i.e. streptokinase, t-PA, accelerated t-PA). Given the 15% reduction in 30-day mortality with ‘accelerated’ tPA as compared with streptokinase in the GUSTO-I trial, the former has become the ‘gold’ standard for pharmacological reperfusion therapy.3 Thus, a sensitivity analysis was performed to evaluate if results of the primary analysis were consistent in the studies that compared PPCI with accelerated tPA.
Results
Of 6763 patients, 3383 were randomized to FL and 3380 to PPCI. The distribution of baseline patient characteristics among patients randomized to FL and PPCI was well balanced, with prior CABG as the exception. (Table 1) Younger, male patients and patients with a history of previous MI, or PCI presented earlier, whereas those with diabetes mellitus appeared to have arrived later, especially after 6 h. Patients with an anterior MI tended to present either very early (0–1 h) or very late (>6 h) after symptom onset. In trials with available data, 3.3% of patients randomized to FL actually receive PPCI, whereas 12.1% of patients randomized to PPCI did not receive the assigned treatment.
Overall, the median presentation delay was 142 (IQR: 91–221) min, which did not differ significantly between FL [143 (91–225) min] and PPCI [140 (91–220) min], P=0.30. Nearly 11% of the 6763 patients were randomized within the first hour after symptom onset, with the majority subsequently randomized between 1 and 3 h after symptom onset (Figure 2). As also shown in Figure 2, the time from randomization to the start of either FL or PPCI was not influenced by the length of time from symptom onset. Given the inherent logistics of the different modalities, the median time to FL was significantly shorter than that of the beginning of PCI [respectively, 19 (10–30) min vs. 76 (61–95) min; P<0.001], which gave an overall PCI-related delay of 55 (37–74) min.
Thirty-day adverse events and the influence of presentation delay
Overall, 6.6% of 6763 patients died within 30 days of randomization (Table 2). According to randomized treatment, the 30-day death rate was 7.9% of FL patients and 5.3% in those randomized to PPCI (P<0.001). In patients randomized to FL, 30-day mortality increased two-fold as the presentation delay increased from less than 1 to over 6 h (P<0.001). A similar, yet non-significant trend was observed in patients assigned to PPCI (P=0.06).
Re-infarction occurred in 6.7% of FL patients and in 2.4% of PPCI patients during 30-day follow-up (P<0.001). Among FL patients, re-infarction occurred in 10.4% of patients enrolled within 1 h after symptom onset, and in 6.3% of patients enrolled at more than 6 h after symptom onset (P=0.007). In those randomized to PPCI, there was no difference in the re-infarction rate at 30 days according to presentation delay (P=0.5).
The combined endpoint of 30-day death and re-infarction occurred in 13.5% of FL patients and in 7.3% of PPCI patients (P<0.001). In both treatment groups, there appeared to be an increasing trend in 30-day death or re-infarction with longer presentation delay; however, this was only statistically significant in the FL group (P<0.001).
Treatment, time to treatment, and 30-day mortality
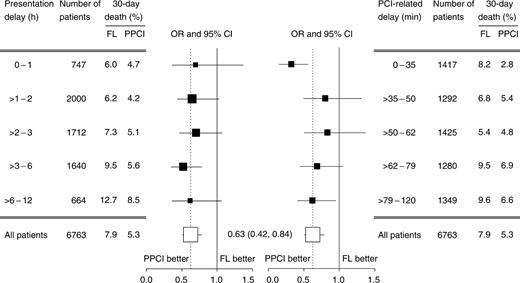
Overall, PPCI patients had a 37% relative lower odds of 30-day mortality than those randomized to fibrinolytic therapy after multi-level covariate adjustment [adjusted OR 0.63; 95% CI (0.42–0.84); P<0.001]. According to presentation delay, the treatment effect consistently favoured PPCI in all the subgroups, and there was no evidence of heterogeneity (pBreslow-Day=0.9) (Figure 3, left panel). The absolute mortality reduction by PPCI increased from 1.3% in patients randomized in the first hour after symptom onset to 4.2% in those randomized after 6 h. Consequently, with increasing delay, the number needed treat to prevent a death, decreased from 77 to 24 patients.
According to quintiles of site-specific PPCI-related delay, the risk of 30-day death was consistently reduced in PPCI patients, and there was evidence of heterogeneity across the quintiles (pBreslow-Day=0.05) (Figure 3, right panel). In particular, PPCI was associated with a 67% reduction in the odds of 30-day mortality when the PPCI-related delay was less than or equal to 35 min and with a 28% reduction in patients with a longer PPCI-related delay (pBreslow-Day=0.004 for the comparison of the first quintile with quintiles 2–5).
Subgroup and sensitivity analyses
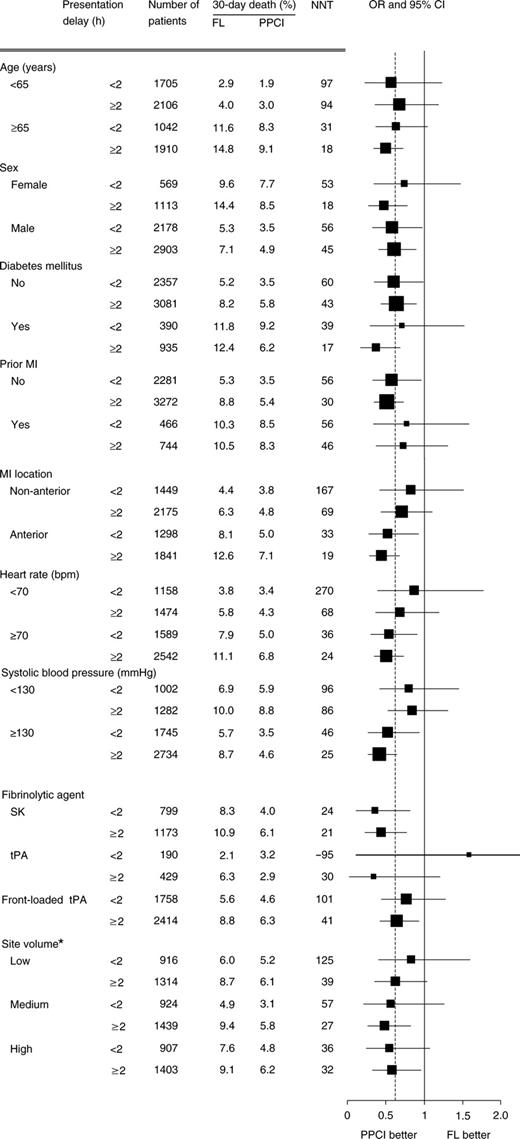
The relationship between relative treatment effect and presentation delay (less than or equal to/greater than 2 h) were examined within selected patient-, hospital-, and study-level characteristics (Figure 4). In general, treatment with PPCI was consistently favoured over FL in the subgroups considered, regardless of presentation delay. The average annual PCI volume did not influence the relative treatment benefit of PPCI over FL. The relative treatment effect appeared to decline with the use of accelerated t-PA and the year of randomization; however, these trends were not statistically significant.
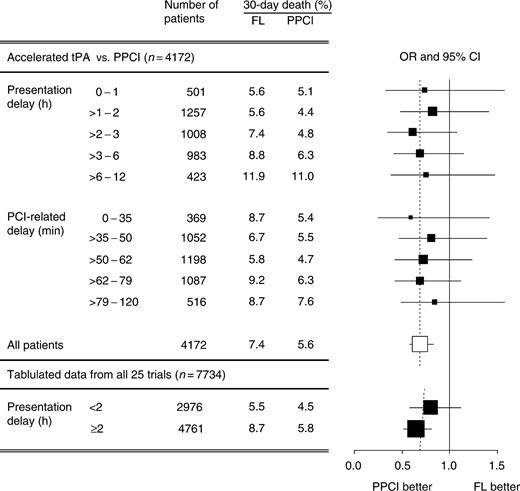
Ten of the 22 studies (n=4172) compared PPCI with accelerated t-PA (Appendix B, Table B1). In these trials, PPCI was associated with a 29% relative reduction in mortality [7.4% FL vs. 5.6% PPCI; adjusted OR: 0.71; 95% CI (0.46–0.98)]. Similar to the primary analysis, there was no evidence of heterogeneity in the treatment effect according to presentation delay (pBreslow-Day=0.9; Figure 5). Also, as seen in the primary analysis, the treatment effect in favour of PPCI was highest in the first quintile of PCI-related delay. The 95% CI, however, were wide and largely overlapping, resulting in lack of heterogeneity (pBreslow-Day=0.3).
Although individual patient data for three of the 25 trials originally identified were not available, tabular data for 980 patients were extracted from published reports and analysed for potential bias based on their exclusion.15,23,28 Overall, a 31% reduction in 30-day death was observed in the PPCI group [7.5% FL vs. 5.3% PPCI; OR: 0.69, 95% CI (0.58–0.83)]. The CAPTIM trial was of particular interest as ∼55% of the 840 patients were randomized within the first 2 h after symptom onset. Despite these additional patients, the relative treatment benefit was still in favour of PPCI, regardless of presentation delay (pBreslow-Day=0.3) (Figure 5). If only accelerated t-PA trials were analysed, the relative benefit was attenuated but still without statistical evidence of heterogeneity (<2 h, OR: 0.98; 95% CI (0.66–1.47); ≥2 h, OR: 0.72; 95% CI (0.54–0.95); pBreslow-Day=0.21).
Discussion
Overall, PPCI was associated with a 37% relative reduction in the odds of 30-day mortality when compared with in-hospital fibrinolytic therapy, which was slightly attenuated to 28% when only accelerated t-PA trials were considered. Although these findings are not unlike those found in previously published meta- and pooled-analyses,11,12,43 this analysis extends beyond these by including individual patient data from trials reported since 1997 and focusing on the importance of time to treatment in the reperfusion debate.
The time-sensitivity of fibrinolytic therapy has been well established by the substantial reduction in mortality observed when fibrinolytic treatment is administered within the ‘golden hour’.5 Yet, regardless of the delay in presentation of the current analysis, treatment with PPCI was uniformly associated with lower mortality relative to FL. These findings were somewhat unexpected given the recently reported evidence. In the CAPTIM trial, for instance, patients randomized within 2 h after symptom onset had a strong trend towards higher 30-day mortality with PPCI when compared with pre-hospital FL [5.7 vs. 2.2%; OR: 2.7; 95% CI (0.94–7.7); P=0.06].18 Although our sensitivity analysis including the tabulated data from the CAPTIM trial suggests that PPCI provides only a small mortality reduction relative to accelerated t-PA in patients randomized within 2 h, this estimate was not adjusted for potential confounders. Also, from statistical point of view, the estimate of treatment effect in this subgroup did not diverge from the estimate in patients randomized after 2 h. Upon more extensive analysis of the CAPTIM trial, several important details are worth noting. First, this trial was designed to prove the clinical superiority of PPCI over pre-hospital FL. Although, 1200 patients were needed to demonstrate a foreseen 44% relative reduction in the composite endpoint of death, non-fatal myocardial re-infarction and non-fatal stroke, only 67% of the target sample size was achieved. As a result, only a trend towards lower event rates after PPCI was observed (6.2 vs. 8.2% events) and the 95% CI of expected and observed treatment effect were largely overlapping. Thus, the CAPTIM findings should be interpreted with caution and in the context of other pieces of evidence. Finally, over two-thirds of the CAPTIM patients allocated to pre-hospital FL underwent PCI within 30 days, which was notably a strong confounder of treatment effect in our pooled analysis. In fact, the pre-hospital FL treatment strategy of the CAPTIM trial closely approximates the strategy of so-called ‘facilitated’ PCI in which patients receive adjunctive pharmacologic treatment during transfer for the intervention. Some evidence suggests that such strategy may result in even better patient outcome than the more traditional PPCI approach.44,45 On the other hand, preliminary analyses of the ASSENT-IV trial demonstrated a higher incidence of major adverse cardiac complications in patients allocated to the strategy of PPCI after pre-treatment with tenecteplase compared with PPCI alone.46 Several trials such as the Combined Abciximab REteplase Stent Study in acute myocardial infarction (CARESS in AMI),47 and the Facilitated Intervention for Enhanced reperfusion Speed to Stop ischemic Events (FINESSE) trial are underway to further test this strategy.
The balance of the treatment effect in the current analysis remained with PPCI when its association with PCI-related delay was examined, particularly if the delay was 35 min or less. In 2001, Kent and colleagues48 presented a ‘back-of-the-envelope’ calculation for the benefit of PPCI over FL in relation to PCI-related delay. Based on a linear meta-regression on published findings from the first 10 randomized trials of PPCI vs. FL, they concluded that the absolute survival benefit of PPCI compared with FL decreased by 1.7% for every additional 10 min PCI-related delay, and thus, a PCI-related delay exceeding 50 min would nullify its benefits. Nallamothu and Bates49 recently repeated the analysis using published data of most of the trials included in our pooled-analysis and reached similar conclusions. Our analysis, however, yielded divergent results which may largely be explained by several methodological differences. First, Kent and Nallamothu presented only absolute treatment effects. Second, the estimated PCI-related delay in their analyses was based on a combination of median and mean values of time-from-onset- to-treatment, time-from-randomization-to-treatment, and time-from-hospitalization-to-treatment, depending on the available data in the separate clinical trial reports. Finally, the results of linear (meta-) regression are susceptible to extreme observations. Although ‘back-of-the-envelope’ calculations based on tabulated data are relatively straightforward and easy to understand, the caveats of such analyses underscores the importance of pooling individual patient data for these analyses.
PCI-related delay is subject to numerous biases related to the modalities themselves, which are distinct from those influencing presentation delays. These biases are often related to the delivery of these treatment strategies by the healthcare system; and thus, PCI delay was determined at the hospital level in the current analysis. This also has the advantage that PCI delay could be estimated in patients randomized to PPCI who ultimately did not undergo this procedure. However, these analyses are limited to the extent that they are inadequately powered to demonstrate effects of a delay in treatment. Hence, the observations in relation to presentation delay should be given more weight than those regarding PCI-related delay.
One of the most salient messages of the current study is the importance of timely treatment in AMI patients. Although the relative mortality reduction by fibrinolytic therapy (relative to control) is often highest in patients treated earliest after symptom onset,5 our data also provides evidence that this is also the case for PPCI, as the relative treatment effects of PCI over FL were not influenced by presentation delay. Secondly, absolute mortality rates in patients undergoing PPCI increased with increasing presentation delay as well as with increasing PCI-related delay. Although we appreciate that these differences may be biased by differences in patient risk profiles, such as the elderly and diabetics who presented later after symptom onset, it is also important to emphasize that time to treatment remained an important outcome determinant after adjustment for baseline characteristics. This observation is consistent with the results from the large (n=27 080) NRMI registry and a smaller (n=1791) registry from The Netherlands, and confirms that timely treatment results in improved outcomes.13,50
Limitations
Several limitations of this analysis should be addressed. First, the selection of trials may be prone to some bias. As stated earlier, all trials published between January 1990 and December 2002 were considered in the search. Although some time has elapsed since then, to our knowledge, no additional trials have been reported.
Another implication of including trials published since 1990 may be the challenge of generalizability to current practice, given the rapid evolution of therapies and overall cardiac care. Although our analysis revealed no variance across the 22 trials, certain characteristics of recent studies deserve comment. Enrolment in DANAMI-2 and C-PORT, which when combined contributed 30% to the total number of patients in this pooled analysis, was prematurely discontinued owing to better outcomes in patients randomized to PPCI, which were largely driven by high re-infarction rates in FL patients or exclusion of procedure-related re-infarctions. Ideally, a large randomized trial enrolling a broad spectrum of AMI patients would be preferable to meta-analyses. A trial with 80% power would need to enrol 4400 patients (2200 patients in each study arm) to detect a 2% mortality difference.51 Enrolling large numbers of patients, however, has proven in the past to be a challenge as indicated by the early termination of such recent trials as DANAMI-2 and C-PORT. Thus, meta- and pooled-analyses, such as this one, provide our best estimate of reperfusion strategies in these patients. Population-based studies such as those from NRMI and others, however, will provide critical evidence as to how the broader AMI population and healthcare system factors will modulate the observations based in the clinical trial setting.
Although the use of individual patient data provided greater analytic flexibility than traditional meta-analyses, additional information on the context of treatment may have been helpful in further elucidating these research questions. Information on the experience (and personal characteristics) of the interventionalist (and/or intervention team), timing of PCI (‘business-hours’ vs. ‘off-hours’), and geographic-, socio-, and economic-related barriers to care is rarely collected by clinical trials, but may have profound implications to the generalizability of the findings which comes with confusing aggregate and individual effects, otherwise known the ‘ecological fallacy’.52
Conclusions and clinical implications
Regardless of the therapeutic strategy, the time expired since the beginning of the coronary occlusion remains central to the reperfusion debate. With this in mind, efforts should be increased to enhance early reperfusion and solutions should involve all stakeholders, from patients to providers to policy-makers.2 Altering public perception of AMI and the importance of seeking early treatment is a complex undertaking which may be overcome through effective education programming among other behaviour-changing approaches.
The ‘real world’ poses logistical and economic challenges to the feasibility of a ‘PPCI-for-all’ approach; however, the benefit of timely treatment as demonstrated in this study underscores the importance of developing a comprehensive and unified approach to improve the delivery of cardiac care in all AMI patients. Unlike the clinical trial setting, disparities in ambulatory care and pre-hospital services, and limited access to tertiary or regional heart centres, both in number of centres and 24-h/7-day capabilities, represent formidable challenges to translating treatment benefit into the general AMI patient population. For example population-based studies have revealed median treatment delays ranging from 42 to 93 min.53–55 Until these gaps are narrowed, FL still remains a viable treatment strategy when timely PPCI is not available. One treatment does not fit all: time matters.
Acknowledgements
This study was supported through a research grant from Boehringer Ingelheim (The Netherlands). The initiation, design and co-ordination of this project, as well as the interpretation and reporting of the findings were entirely independent of this source.
Conflict of interest: none declared in addition to those declared in the primary publications of the individual trials.
Appendix A. PCAT-2 Trialists' Collaborative Group
Study Chairs: E Boersma (Erasmus MC, Rotterdam, The Netherlands), RJ Simes (NHMRC Clinical Trials Centre, Sydney, Australia), CL Grines (Beaumont Hospital, Royal Oak, MI, USA).
Writing Committee: E Boersma, CM Westerhout (Rotterdam, The Netherlands; Edmonton, Canada), RJ Simes, WD Weaver (Detroit, MI, USA), HR Andersen (Aarhus, Denmark), PW Armstrong (Edmonton, Canada), C Granger (Durham, NC, USA), CL Grines, F Zijlstra (Groningen, The Netherlands), P Widimsky (Prague, Czech Republic).
Trialists: Zwolle studies: MJ de Boer (Zwolle, The Netherlands), F Zijlstra (Groningen, The Netherlands); E Ribeiro (Sao Paulo, Brazil); L Grinfeld (Buenos Aires, Argentina); F Akhras (London, UK); S Kedev (Skopje, Macedonia); PRAGUE studies: P Widimsky (Prague, Czech Republic); MA DeWood (Spokane, WA, USA); Mayo Trial: RJ Gibbons (Rochester, MN, USA); PAMI and AIR-PAMI: CL Grines (Royal Oak, MI, USA); GUSTO-IIb: CB Granger, R Califf (Durham, NC, USA), PW Armstrong (Edmonton, Canada), and RJ Simes (Sydney, Australia); JIMI: H Aoki (Morioka, Japan); J Morais (Leiria, Portugal); F Ribichini (Cuneo, Italy); E Garcia (Madrid, Spain); LIMI: F Bär (Maastricht, The Netherlands); STAT: MR LeMay (Ottawa, Canada); STOPAMI studies: A Kastrati, A Schömig (München, Germany); C-PORT: T Aversano (Baltimore, MD, USA); DANAMI-2: HR Anderson, TT Nielsen (Aarhus, Denmark).
Study support: L Barnes, C Pollicino (NHMRC Clinical Trials Centre, Sydney, Australia).
Appendix B
Design characteristics and 30-day mortality of the 25 randomized clinical trials (PPCI vs. FL) identified in the literature search
| Trial . | Patient population . | Symptom onset (h) . | FL . | PPCI . | |||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Number of patients . | Agent . | 30-day death (%) . | Number of patients . | Stent used . | GP IIb/IIIa used . | 30-day death (%) . |
| Streptokinase agent | |||||||||
| Zijlstra et al.20 | ST↑, ≤75 years | <6 | 149 | SK | 7.4 | 152 | No | No | 1.3 |
| Ribeiro et al.21 | ST↑, <75 years | <6 | 50 | SK | 6.0 | 50 | No | No | 2.0 |
| Grinfeld et al.22 | ST↑ | <12 | 58 | SK | 9.3 | 54 | No | No | 13.8 |
| Zijlstra et al.23 | ST↑, Low risk | <6 | 53 | SK | 2.1 | 47 | No | No | 1.9 |
| Akhras et al.24 | ST↑ | <12 | 45 | SK | 0.0 | 42 | No | No | 8.9 |
| Kedev et al.25 | ST↑ | <12 | 67 | SK | 10.4 | 68 | No | No | 2.9 |
| Prague-116 | ST↑, LBBB | <6 | 99 | SK | 14.1 | 101 | Yes | No | 6.9 |
| De Boer et al.26 | ST↑, >75 years | <6 | 41 | SK | 22.0 | 46 | Yes | No | 6.5 |
| Prague-217 | ST↑ | <12 | 421 | SK | 10.0 | 429 | Yes | Yes | 6.8 |
| Fibrin-specific agent | |||||||||
| DeWood et al.a27 | Anterior MI, ST↑, ≤75 years | <12 | 44 | Duteplase | 4.5 | 46 | No | No | 6.5 |
| Gibbons et al.28 | ST↑, <80 years | <12 | 56 | Duteplase | 3.6 | 47 | No | No | 4.3 |
| Grines et al.29 | ST↑ | <12 | 200 | t-PA | 6.5 | 195 | No | No | 3.1 |
| GUSTO IIb30 | ST↑, LBBB | <12 | 573 | t-PAc | 7.0 | 565 | No | No | 5.7 |
| JIMI31 | ST↑, <80 years | <6 | 62 | t-PA | 1.6 | 59 | No | No | 1.7 |
| Morais et al.a32 | Anterior MI, <70 years | <12 | 25 | t-PAc | 12.0 | 25 | No | No | 16.0 |
| Ribichini et al.33 | Inferior/anterior ST↓, <80 years | <6 | 55 | t-PAc | 5.5 | 55 | No | No | 1.8 |
| Garcia et al.34 | Anterior MI | 5 | 94 | t-PAc | 10.6 | 95 | No | No | 3.2 |
| LIMI35 | ST↑, <80 years | <6 | 75 | t-PAc | 6.7 | 75 | Yes | No | 5.3 |
| STAT36 | ST↑, LBBB | <12 | 61 | t-PAc | 3.3 | 62 | Yes | Yes | 3.2 |
| STOPAMI-137 | ST↑ | <12 | 69 | t-PAc | 7.2 | 71 | Yes | Yes | 4.2 |
| AIR PAMI38 | Anterior MI, >70 years | <12 | 66 | t-PAc | 12.1 | 71 | Yes | Yes | 8.5 |
| C-PORT39 | ST↑ | <12 | 226 | t-PAc | 7.1 | 225 | Yes | Yes | 5.3 |
| DANAMI-215 | ST↑ | <12 | 782 | t-PAc | 7.8 | 790 | Yes | NA | 6.6 |
| STOPAMI-240 | ST↑, LBBB | <12 | 81 | t-PAc | 6.2 | 81 | Yes | Yes | 2.5 |
| Pre-hospital FL | |||||||||
| CAPTIMb19 | ST↑ | <6 | 419 | t-PAc | 3.8 | 421 | Yes | Yes | 4.8 |
| Trial . | Patient population . | Symptom onset (h) . | FL . | PPCI . | |||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Number of patients . | Agent . | 30-day death (%) . | Number of patients . | Stent used . | GP IIb/IIIa used . | 30-day death (%) . |
| Streptokinase agent | |||||||||
| Zijlstra et al.20 | ST↑, ≤75 years | <6 | 149 | SK | 7.4 | 152 | No | No | 1.3 |
| Ribeiro et al.21 | ST↑, <75 years | <6 | 50 | SK | 6.0 | 50 | No | No | 2.0 |
| Grinfeld et al.22 | ST↑ | <12 | 58 | SK | 9.3 | 54 | No | No | 13.8 |
| Zijlstra et al.23 | ST↑, Low risk | <6 | 53 | SK | 2.1 | 47 | No | No | 1.9 |
| Akhras et al.24 | ST↑ | <12 | 45 | SK | 0.0 | 42 | No | No | 8.9 |
| Kedev et al.25 | ST↑ | <12 | 67 | SK | 10.4 | 68 | No | No | 2.9 |
| Prague-116 | ST↑, LBBB | <6 | 99 | SK | 14.1 | 101 | Yes | No | 6.9 |
| De Boer et al.26 | ST↑, >75 years | <6 | 41 | SK | 22.0 | 46 | Yes | No | 6.5 |
| Prague-217 | ST↑ | <12 | 421 | SK | 10.0 | 429 | Yes | Yes | 6.8 |
| Fibrin-specific agent | |||||||||
| DeWood et al.a27 | Anterior MI, ST↑, ≤75 years | <12 | 44 | Duteplase | 4.5 | 46 | No | No | 6.5 |
| Gibbons et al.28 | ST↑, <80 years | <12 | 56 | Duteplase | 3.6 | 47 | No | No | 4.3 |
| Grines et al.29 | ST↑ | <12 | 200 | t-PA | 6.5 | 195 | No | No | 3.1 |
| GUSTO IIb30 | ST↑, LBBB | <12 | 573 | t-PAc | 7.0 | 565 | No | No | 5.7 |
| JIMI31 | ST↑, <80 years | <6 | 62 | t-PA | 1.6 | 59 | No | No | 1.7 |
| Morais et al.a32 | Anterior MI, <70 years | <12 | 25 | t-PAc | 12.0 | 25 | No | No | 16.0 |
| Ribichini et al.33 | Inferior/anterior ST↓, <80 years | <6 | 55 | t-PAc | 5.5 | 55 | No | No | 1.8 |
| Garcia et al.34 | Anterior MI | 5 | 94 | t-PAc | 10.6 | 95 | No | No | 3.2 |
| LIMI35 | ST↑, <80 years | <6 | 75 | t-PAc | 6.7 | 75 | Yes | No | 5.3 |
| STAT36 | ST↑, LBBB | <12 | 61 | t-PAc | 3.3 | 62 | Yes | Yes | 3.2 |
| STOPAMI-137 | ST↑ | <12 | 69 | t-PAc | 7.2 | 71 | Yes | Yes | 4.2 |
| AIR PAMI38 | Anterior MI, >70 years | <12 | 66 | t-PAc | 12.1 | 71 | Yes | Yes | 8.5 |
| C-PORT39 | ST↑ | <12 | 226 | t-PAc | 7.1 | 225 | Yes | Yes | 5.3 |
| DANAMI-215 | ST↑ | <12 | 782 | t-PAc | 7.8 | 790 | Yes | NA | 6.6 |
| STOPAMI-240 | ST↑, LBBB | <12 | 81 | t-PAc | 6.2 | 81 | Yes | Yes | 2.5 |
| Pre-hospital FL | |||||||||
| CAPTIMb19 | ST↑ | <6 | 419 | t-PAc | 3.8 | 421 | Yes | Yes | 4.8 |
GP, glycoprotein IIb/IIIa inhibitor; LBBB, left bunch branch block; SK, streptokinase; ST↑, ST-segment elevation; ST↓, ST-segment depression.
aExcluded from primary analysis because of non-availability of individual patient data.
bExcluded from primary analysis because pre-hospital FL.
cAccelerated t-PA.
Design characteristics and 30-day mortality of the 25 randomized clinical trials (PPCI vs. FL) identified in the literature search
| Trial . | Patient population . | Symptom onset (h) . | FL . | PPCI . | |||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Number of patients . | Agent . | 30-day death (%) . | Number of patients . | Stent used . | GP IIb/IIIa used . | 30-day death (%) . |
| Streptokinase agent | |||||||||
| Zijlstra et al.20 | ST↑, ≤75 years | <6 | 149 | SK | 7.4 | 152 | No | No | 1.3 |
| Ribeiro et al.21 | ST↑, <75 years | <6 | 50 | SK | 6.0 | 50 | No | No | 2.0 |
| Grinfeld et al.22 | ST↑ | <12 | 58 | SK | 9.3 | 54 | No | No | 13.8 |
| Zijlstra et al.23 | ST↑, Low risk | <6 | 53 | SK | 2.1 | 47 | No | No | 1.9 |
| Akhras et al.24 | ST↑ | <12 | 45 | SK | 0.0 | 42 | No | No | 8.9 |
| Kedev et al.25 | ST↑ | <12 | 67 | SK | 10.4 | 68 | No | No | 2.9 |
| Prague-116 | ST↑, LBBB | <6 | 99 | SK | 14.1 | 101 | Yes | No | 6.9 |
| De Boer et al.26 | ST↑, >75 years | <6 | 41 | SK | 22.0 | 46 | Yes | No | 6.5 |
| Prague-217 | ST↑ | <12 | 421 | SK | 10.0 | 429 | Yes | Yes | 6.8 |
| Fibrin-specific agent | |||||||||
| DeWood et al.a27 | Anterior MI, ST↑, ≤75 years | <12 | 44 | Duteplase | 4.5 | 46 | No | No | 6.5 |
| Gibbons et al.28 | ST↑, <80 years | <12 | 56 | Duteplase | 3.6 | 47 | No | No | 4.3 |
| Grines et al.29 | ST↑ | <12 | 200 | t-PA | 6.5 | 195 | No | No | 3.1 |
| GUSTO IIb30 | ST↑, LBBB | <12 | 573 | t-PAc | 7.0 | 565 | No | No | 5.7 |
| JIMI31 | ST↑, <80 years | <6 | 62 | t-PA | 1.6 | 59 | No | No | 1.7 |
| Morais et al.a32 | Anterior MI, <70 years | <12 | 25 | t-PAc | 12.0 | 25 | No | No | 16.0 |
| Ribichini et al.33 | Inferior/anterior ST↓, <80 years | <6 | 55 | t-PAc | 5.5 | 55 | No | No | 1.8 |
| Garcia et al.34 | Anterior MI | 5 | 94 | t-PAc | 10.6 | 95 | No | No | 3.2 |
| LIMI35 | ST↑, <80 years | <6 | 75 | t-PAc | 6.7 | 75 | Yes | No | 5.3 |
| STAT36 | ST↑, LBBB | <12 | 61 | t-PAc | 3.3 | 62 | Yes | Yes | 3.2 |
| STOPAMI-137 | ST↑ | <12 | 69 | t-PAc | 7.2 | 71 | Yes | Yes | 4.2 |
| AIR PAMI38 | Anterior MI, >70 years | <12 | 66 | t-PAc | 12.1 | 71 | Yes | Yes | 8.5 |
| C-PORT39 | ST↑ | <12 | 226 | t-PAc | 7.1 | 225 | Yes | Yes | 5.3 |
| DANAMI-215 | ST↑ | <12 | 782 | t-PAc | 7.8 | 790 | Yes | NA | 6.6 |
| STOPAMI-240 | ST↑, LBBB | <12 | 81 | t-PAc | 6.2 | 81 | Yes | Yes | 2.5 |
| Pre-hospital FL | |||||||||
| CAPTIMb19 | ST↑ | <6 | 419 | t-PAc | 3.8 | 421 | Yes | Yes | 4.8 |
| Trial . | Patient population . | Symptom onset (h) . | FL . | PPCI . | |||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Number of patients . | Agent . | 30-day death (%) . | Number of patients . | Stent used . | GP IIb/IIIa used . | 30-day death (%) . |
| Streptokinase agent | |||||||||
| Zijlstra et al.20 | ST↑, ≤75 years | <6 | 149 | SK | 7.4 | 152 | No | No | 1.3 |
| Ribeiro et al.21 | ST↑, <75 years | <6 | 50 | SK | 6.0 | 50 | No | No | 2.0 |
| Grinfeld et al.22 | ST↑ | <12 | 58 | SK | 9.3 | 54 | No | No | 13.8 |
| Zijlstra et al.23 | ST↑, Low risk | <6 | 53 | SK | 2.1 | 47 | No | No | 1.9 |
| Akhras et al.24 | ST↑ | <12 | 45 | SK | 0.0 | 42 | No | No | 8.9 |
| Kedev et al.25 | ST↑ | <12 | 67 | SK | 10.4 | 68 | No | No | 2.9 |
| Prague-116 | ST↑, LBBB | <6 | 99 | SK | 14.1 | 101 | Yes | No | 6.9 |
| De Boer et al.26 | ST↑, >75 years | <6 | 41 | SK | 22.0 | 46 | Yes | No | 6.5 |
| Prague-217 | ST↑ | <12 | 421 | SK | 10.0 | 429 | Yes | Yes | 6.8 |
| Fibrin-specific agent | |||||||||
| DeWood et al.a27 | Anterior MI, ST↑, ≤75 years | <12 | 44 | Duteplase | 4.5 | 46 | No | No | 6.5 |
| Gibbons et al.28 | ST↑, <80 years | <12 | 56 | Duteplase | 3.6 | 47 | No | No | 4.3 |
| Grines et al.29 | ST↑ | <12 | 200 | t-PA | 6.5 | 195 | No | No | 3.1 |
| GUSTO IIb30 | ST↑, LBBB | <12 | 573 | t-PAc | 7.0 | 565 | No | No | 5.7 |
| JIMI31 | ST↑, <80 years | <6 | 62 | t-PA | 1.6 | 59 | No | No | 1.7 |
| Morais et al.a32 | Anterior MI, <70 years | <12 | 25 | t-PAc | 12.0 | 25 | No | No | 16.0 |
| Ribichini et al.33 | Inferior/anterior ST↓, <80 years | <6 | 55 | t-PAc | 5.5 | 55 | No | No | 1.8 |
| Garcia et al.34 | Anterior MI | 5 | 94 | t-PAc | 10.6 | 95 | No | No | 3.2 |
| LIMI35 | ST↑, <80 years | <6 | 75 | t-PAc | 6.7 | 75 | Yes | No | 5.3 |
| STAT36 | ST↑, LBBB | <12 | 61 | t-PAc | 3.3 | 62 | Yes | Yes | 3.2 |
| STOPAMI-137 | ST↑ | <12 | 69 | t-PAc | 7.2 | 71 | Yes | Yes | 4.2 |
| AIR PAMI38 | Anterior MI, >70 years | <12 | 66 | t-PAc | 12.1 | 71 | Yes | Yes | 8.5 |
| C-PORT39 | ST↑ | <12 | 226 | t-PAc | 7.1 | 225 | Yes | Yes | 5.3 |
| DANAMI-215 | ST↑ | <12 | 782 | t-PAc | 7.8 | 790 | Yes | NA | 6.6 |
| STOPAMI-240 | ST↑, LBBB | <12 | 81 | t-PAc | 6.2 | 81 | Yes | Yes | 2.5 |
| Pre-hospital FL | |||||||||
| CAPTIMb19 | ST↑ | <6 | 419 | t-PAc | 3.8 | 421 | Yes | Yes | 4.8 |
GP, glycoprotein IIb/IIIa inhibitor; LBBB, left bunch branch block; SK, streptokinase; ST↑, ST-segment elevation; ST↓, ST-segment depression.
aExcluded from primary analysis because of non-availability of individual patient data.
bExcluded from primary analysis because pre-hospital FL.
cAccelerated t-PA.
Figure 1 Flowchart of trial search and selection for the pooled analysis.
Figure 2 Distribution of patients (bars) and median (25th, 75th percentile) time to treatment [FL (▪)] or [PPCI (▪)] according to presentation delay and study treatment.
Figure 3 OR and 95% CI for 30-day death in patients randomized to PPCI when compared with FL according to presentation delay (left panel) and PCI-related delay (right panel). OR were adjusted for patient-, hospital-, and study-level covariates.
Figure 4 Subgroup analysis of selected patient-, study-, and site-level characteristics. NNT (number needed to treat): the number of patients who need to be treated in order to prevent a death. OR were adjusted for patient-, hospital-, and study-level covariates. *Site volume on-study only, classified by the number of patients randomized to percutaneous transluminal coronary angioplasty per site per year: low, <10 patients/site/year; medium, 10–23 patients/site/year; high, ≥24 patients/site/year.
Figure 5 Sensitivity analyses of the use of accelerated t-PA and of the inclusion of trials with tabular data. OR were adjusted for patient-, hospital-, and study-level covariates.
Baseline characteristics of the study population according to study treatment and presentation delay
| . | FL . | PPCI . | Presentation delay . | ||||
|---|---|---|---|---|---|---|---|
| . | . | . | 0–1 h . | >1–2 h . | >2–3 h . | >3–6 h . | >6 h . |
| n | 3383 | 3380 | 747 | 2000 | 1712 | 1640 | 664 |
| Demographics and co-morbidities | |||||||
| Age (years)a | 62 (53–71) | 63 (53–71) | 60 (52–68) | 61 (53–70) | 63 (54–71) | 65 (55–73) | 65 (54–74)c |
| Male (%) | 73.2 | 72.6 | 76.6 | 74.8 | 73.2 | 69.5 | 70.6c |
| Diabetes mellitus (%) | 12.4 | 13.5 | 11.1 | 9.8 | 13.1 | 14.7 | 20.0c |
| Previous MI (%) | 13.2 | 12.5 | 14.1 | 13.5 | 12.6 | 11.9 | 12.5c |
| History of PCI (%) | 4.0 | 3.6 | 3.5 | 5.1 | 3.3 | 2.9 | 3.6d |
| History of CABG (%) | 2.1 | 1.2b | 1.3 | 2.1 | 1.5 | 1.6 | 1.4 |
| Clinical examination | |||||||
| Body weight (kg)a | 79 (75–80) | 79 (75–80) | 79 (77–82) | 79 (75–80) | 79 (75–80) | 79 (73–80) | 79 (72–80) |
| Systolic blood pressure (mmHg)a | 133 (120–145) | 133 (120–145) | 133 (115–140) | 133 (120–140) | 133 (120–146) | 133 (120–148) | 133 (120–150) |
| Heart rate (bpm)a | 76 (66–82) | 76 (66–84) | 76 (64–80) | 76 (63–80) | 76 (67–84) | 76 (68–84) | 76 (70–90) |
| Anterior MI (%) | 45.8 | 46.5 | 49.4 | 46.2 | 43.1 | 44.6 | 52.4 |
| . | FL . | PPCI . | Presentation delay . | ||||
|---|---|---|---|---|---|---|---|
| . | . | . | 0–1 h . | >1–2 h . | >2–3 h . | >3–6 h . | >6 h . |
| n | 3383 | 3380 | 747 | 2000 | 1712 | 1640 | 664 |
| Demographics and co-morbidities | |||||||
| Age (years)a | 62 (53–71) | 63 (53–71) | 60 (52–68) | 61 (53–70) | 63 (54–71) | 65 (55–73) | 65 (54–74)c |
| Male (%) | 73.2 | 72.6 | 76.6 | 74.8 | 73.2 | 69.5 | 70.6c |
| Diabetes mellitus (%) | 12.4 | 13.5 | 11.1 | 9.8 | 13.1 | 14.7 | 20.0c |
| Previous MI (%) | 13.2 | 12.5 | 14.1 | 13.5 | 12.6 | 11.9 | 12.5c |
| History of PCI (%) | 4.0 | 3.6 | 3.5 | 5.1 | 3.3 | 2.9 | 3.6d |
| History of CABG (%) | 2.1 | 1.2b | 1.3 | 2.1 | 1.5 | 1.6 | 1.4 |
| Clinical examination | |||||||
| Body weight (kg)a | 79 (75–80) | 79 (75–80) | 79 (77–82) | 79 (75–80) | 79 (75–80) | 79 (73–80) | 79 (72–80) |
| Systolic blood pressure (mmHg)a | 133 (120–145) | 133 (120–145) | 133 (115–140) | 133 (120–140) | 133 (120–146) | 133 (120–148) | 133 (120–150) |
| Heart rate (bpm)a | 76 (66–82) | 76 (66–84) | 76 (64–80) | 76 (63–80) | 76 (67–84) | 76 (68–84) | 76 (70–90) |
| Anterior MI (%) | 45.8 | 46.5 | 49.4 | 46.2 | 43.1 | 44.6 | 52.4 |
aContinuous data are presented as median values (IQR).
bDifference between patients assigned to FL or PPCI, P<0.05.
cDifference across subgroups according to time from symptom onset to randomization, P<0.05.
dDifference across subgroups according to time from symptom onset to randomization, P<0.001.
Baseline characteristics of the study population according to study treatment and presentation delay
| . | FL . | PPCI . | Presentation delay . | ||||
|---|---|---|---|---|---|---|---|
| . | . | . | 0–1 h . | >1–2 h . | >2–3 h . | >3–6 h . | >6 h . |
| n | 3383 | 3380 | 747 | 2000 | 1712 | 1640 | 664 |
| Demographics and co-morbidities | |||||||
| Age (years)a | 62 (53–71) | 63 (53–71) | 60 (52–68) | 61 (53–70) | 63 (54–71) | 65 (55–73) | 65 (54–74)c |
| Male (%) | 73.2 | 72.6 | 76.6 | 74.8 | 73.2 | 69.5 | 70.6c |
| Diabetes mellitus (%) | 12.4 | 13.5 | 11.1 | 9.8 | 13.1 | 14.7 | 20.0c |
| Previous MI (%) | 13.2 | 12.5 | 14.1 | 13.5 | 12.6 | 11.9 | 12.5c |
| History of PCI (%) | 4.0 | 3.6 | 3.5 | 5.1 | 3.3 | 2.9 | 3.6d |
| History of CABG (%) | 2.1 | 1.2b | 1.3 | 2.1 | 1.5 | 1.6 | 1.4 |
| Clinical examination | |||||||
| Body weight (kg)a | 79 (75–80) | 79 (75–80) | 79 (77–82) | 79 (75–80) | 79 (75–80) | 79 (73–80) | 79 (72–80) |
| Systolic blood pressure (mmHg)a | 133 (120–145) | 133 (120–145) | 133 (115–140) | 133 (120–140) | 133 (120–146) | 133 (120–148) | 133 (120–150) |
| Heart rate (bpm)a | 76 (66–82) | 76 (66–84) | 76 (64–80) | 76 (63–80) | 76 (67–84) | 76 (68–84) | 76 (70–90) |
| Anterior MI (%) | 45.8 | 46.5 | 49.4 | 46.2 | 43.1 | 44.6 | 52.4 |
| . | FL . | PPCI . | Presentation delay . | ||||
|---|---|---|---|---|---|---|---|
| . | . | . | 0–1 h . | >1–2 h . | >2–3 h . | >3–6 h . | >6 h . |
| n | 3383 | 3380 | 747 | 2000 | 1712 | 1640 | 664 |
| Demographics and co-morbidities | |||||||
| Age (years)a | 62 (53–71) | 63 (53–71) | 60 (52–68) | 61 (53–70) | 63 (54–71) | 65 (55–73) | 65 (54–74)c |
| Male (%) | 73.2 | 72.6 | 76.6 | 74.8 | 73.2 | 69.5 | 70.6c |
| Diabetes mellitus (%) | 12.4 | 13.5 | 11.1 | 9.8 | 13.1 | 14.7 | 20.0c |
| Previous MI (%) | 13.2 | 12.5 | 14.1 | 13.5 | 12.6 | 11.9 | 12.5c |
| History of PCI (%) | 4.0 | 3.6 | 3.5 | 5.1 | 3.3 | 2.9 | 3.6d |
| History of CABG (%) | 2.1 | 1.2b | 1.3 | 2.1 | 1.5 | 1.6 | 1.4 |
| Clinical examination | |||||||
| Body weight (kg)a | 79 (75–80) | 79 (75–80) | 79 (77–82) | 79 (75–80) | 79 (75–80) | 79 (73–80) | 79 (72–80) |
| Systolic blood pressure (mmHg)a | 133 (120–145) | 133 (120–145) | 133 (115–140) | 133 (120–140) | 133 (120–146) | 133 (120–148) | 133 (120–150) |
| Heart rate (bpm)a | 76 (66–82) | 76 (66–84) | 76 (64–80) | 76 (63–80) | 76 (67–84) | 76 (68–84) | 76 (70–90) |
| Anterior MI (%) | 45.8 | 46.5 | 49.4 | 46.2 | 43.1 | 44.6 | 52.4 |
aContinuous data are presented as median values (IQR).
bDifference between patients assigned to FL or PPCI, P<0.05.
cDifference across subgroups according to time from symptom onset to randomization, P<0.05.
dDifference across subgroups according to time from symptom onset to randomization, P<0.001.
Adverse events at 30 days according to presentation delay
| . | Overall . | Presentation delay . | ||||
|---|---|---|---|---|---|---|
| . | . | 0–1 h . | >1–2 h . | >2–3 h . | >3–6 h . | >6 h . |
| Fibrinolysis, n | 3383 | 368 | 997 | 818 | 876 | 324 |
| Death (%) | 7.9 | 6.0 | 6.2 | 7.3 | 9.5 | 12.7b |
| Re-MI (%) | 6.7 | 10.4 | 4.9 | 7.4 | 6.9 | 6.3a |
| Death or re-MI (%) | 13.5 | 14.9 | 10.4 | 13.6 | 15.0 | 17.6a |
| Stroke (%) | 2.2 | 4.0 | 0.8 | 1.0 | 5.2 | 0.0 |
| PPCI, n | 3380 | 379 | 1003 | 894 | 764 | 340 |
| Death (%) | 5.3 | 4.7 | 4.2 | 5.1 | 5.6 | 8.5a |
| Re-MI (%) | 2.4 | 1.6 | 2.9 | 2.3 | 2.0 | 3.0 |
| Death or re-MI (%) | 7.3 | 6.1 | 6.8 | 7.4 | 7.2 | 10.3 |
| Stroke (%) | 0.5 | 0.0 | 0.8 | 0.0 | 1.0 | 0.0 |
| . | Overall . | Presentation delay . | ||||
|---|---|---|---|---|---|---|
| . | . | 0–1 h . | >1–2 h . | >2–3 h . | >3–6 h . | >6 h . |
| Fibrinolysis, n | 3383 | 368 | 997 | 818 | 876 | 324 |
| Death (%) | 7.9 | 6.0 | 6.2 | 7.3 | 9.5 | 12.7b |
| Re-MI (%) | 6.7 | 10.4 | 4.9 | 7.4 | 6.9 | 6.3a |
| Death or re-MI (%) | 13.5 | 14.9 | 10.4 | 13.6 | 15.0 | 17.6a |
| Stroke (%) | 2.2 | 4.0 | 0.8 | 1.0 | 5.2 | 0.0 |
| PPCI, n | 3380 | 379 | 1003 | 894 | 764 | 340 |
| Death (%) | 5.3 | 4.7 | 4.2 | 5.1 | 5.6 | 8.5a |
| Re-MI (%) | 2.4 | 1.6 | 2.9 | 2.3 | 2.0 | 3.0 |
| Death or re-MI (%) | 7.3 | 6.1 | 6.8 | 7.4 | 7.2 | 10.3 |
| Stroke (%) | 0.5 | 0.0 | 0.8 | 0.0 | 1.0 | 0.0 |
aDifference across subgroups according to presentation delay, P<0.05.
bDifference across subgroups according to presentation delay, P<0.001.
Adverse events at 30 days according to presentation delay
| . | Overall . | Presentation delay . | ||||
|---|---|---|---|---|---|---|
| . | . | 0–1 h . | >1–2 h . | >2–3 h . | >3–6 h . | >6 h . |
| Fibrinolysis, n | 3383 | 368 | 997 | 818 | 876 | 324 |
| Death (%) | 7.9 | 6.0 | 6.2 | 7.3 | 9.5 | 12.7b |
| Re-MI (%) | 6.7 | 10.4 | 4.9 | 7.4 | 6.9 | 6.3a |
| Death or re-MI (%) | 13.5 | 14.9 | 10.4 | 13.6 | 15.0 | 17.6a |
| Stroke (%) | 2.2 | 4.0 | 0.8 | 1.0 | 5.2 | 0.0 |
| PPCI, n | 3380 | 379 | 1003 | 894 | 764 | 340 |
| Death (%) | 5.3 | 4.7 | 4.2 | 5.1 | 5.6 | 8.5a |
| Re-MI (%) | 2.4 | 1.6 | 2.9 | 2.3 | 2.0 | 3.0 |
| Death or re-MI (%) | 7.3 | 6.1 | 6.8 | 7.4 | 7.2 | 10.3 |
| Stroke (%) | 0.5 | 0.0 | 0.8 | 0.0 | 1.0 | 0.0 |
| . | Overall . | Presentation delay . | ||||
|---|---|---|---|---|---|---|
| . | . | 0–1 h . | >1–2 h . | >2–3 h . | >3–6 h . | >6 h . |
| Fibrinolysis, n | 3383 | 368 | 997 | 818 | 876 | 324 |
| Death (%) | 7.9 | 6.0 | 6.2 | 7.3 | 9.5 | 12.7b |
| Re-MI (%) | 6.7 | 10.4 | 4.9 | 7.4 | 6.9 | 6.3a |
| Death or re-MI (%) | 13.5 | 14.9 | 10.4 | 13.6 | 15.0 | 17.6a |
| Stroke (%) | 2.2 | 4.0 | 0.8 | 1.0 | 5.2 | 0.0 |
| PPCI, n | 3380 | 379 | 1003 | 894 | 764 | 340 |
| Death (%) | 5.3 | 4.7 | 4.2 | 5.1 | 5.6 | 8.5a |
| Re-MI (%) | 2.4 | 1.6 | 2.9 | 2.3 | 2.0 | 3.0 |
| Death or re-MI (%) | 7.3 | 6.1 | 6.8 | 7.4 | 7.2 | 10.3 |
| Stroke (%) | 0.5 | 0.0 | 0.8 | 0.0 | 1.0 | 0.0 |
aDifference across subgroups according to presentation delay, P<0.05.
bDifference across subgroups according to presentation delay, P<0.001.
References
Armstrong PW, Welsh RC. Tailoring therapy to best suit ST-segment elevation myocardial infarction: searching for the right fit.
The GUSTO Angiographic Investigators. The effects of tissue plasminogen activator, streptokinase, or both on coronary-artery patency, ventricular function, survival after acute myocardial infarction.
Fibrinolytic Therapy Trialists' (FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction: colloborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients.
Boersma E, Maas AC, Deckers JW, Simoons ML. Early thrombolytic treatment in acute myocardial infarction: reappraisal of the golden hour.
Meijer A, Verheugt FW, Werter CJ, Lie KI, van der Pol JM, van Eenige MJ. Aspirin vs. coumadin in the prevention of reocclusion and recurrent ischemia after successful thrombolysis: a prospective placebo-controlled angiographic study. Results of the APRICOT Study.
Grines CL. Should thrombolysis or primary angioplasty be the treatment of choice for acute myocardial infarction? Primary angioplasty—the strategy of choice.
Simes RJ, Topol EJ, Holmes DR Jr, White HD, Rutsch WR, Vahanian A, Simoons ML, Morris D, Betriu A, Califf RM. Link between the angiographic substudy and mortality outcomes in a large randomized trial of myocardial reperfusion. Importance of early and complete infarct artery reperfusion. GUSTO-I Investigators.
Kotani J, Mintz GS, Pregowski J, Kalinczuk L, Pichard AD, Satler LF, Suddath WO, Waksman R, Weissman NJ. Volumetric intravascular ultrasound evidence that distal embolization during acute infarct intervention contributes to inadequate myocardial perfusion grade.
Henriques JP, Zijlstra F, Ottervanger JP, de Boer MJ, van't Hof AW, Hoorntje JC, Suryapranata H. Incidence and clinical significance of distal embolization during primary angioplasty for acute myocardial infarction.
Keeley EC, Boura JA, Grines CL. Primary angioplasty vs. intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials.
Weaver WD, Simes RJ, Betriu A, Grines CL, Zijlstra F, Garcia E, Grinfeld L, Gibbons RJ, Ribeiro EE, DeWood MA, Ribichini F. Comparison of primary coronary angioplasty and intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review.
Cannon CP, Gibson CM, Lambrew CT, Shoultz DA, Levy D, French WJ, Gore JM, Weaver WD, Rogers WJ, Tiefenbrunn AJ. Relationship of symptom-onset-to-balloon time and door-to-balloon time with mortality in patients undergoing angioplasty for acute myocardial infarction.
Nallamothu BK, Bates ER, Herrin J, Wang Y, Bradley EH, Krumholz HM. Times to treatment in transfer patients undergoing primary percutaneous coronary intervention in the United States: National Registry of Myocardial Infarction (NRMI)-3/4 analysis.
Andersen HR, Nielsen TT, Rasmussen K, Thuesen L, Kelbaek H, Thayssen P, Abildgaard U, Pedersen F, Madsen JK, Grande P, Villadsen AB, Krusell LR, Haghfelt T, Lomholt P, Husted SE, Vigholt E, Kjaergard HK, Mortensen LS, DANAMI-2 Investigators. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction.
Widimsky P, Groch L, Zelizko M, Aschermann M, Bednar F, Suryapranata H. Multicentre randomized trial comparing transport to primary angioplasty vs. immediate thrombolysis vs. combined strategy for patients with acute myocardial infarction presenting to a community hospital without a catheterization laboratory. The PRAGUE study.
Widimsky P, Budesinsky T, Vorac D, Groch L, Zelizko M, Aschermann M, Branny M, St'asek J, Formanek P, ‘PRAGUE’ Study Group. Long distance transport for primary angioplasty vs. immediate thrombolysis in acute myocardial infarction. Final results of the randomized national multicentre trial—PRAGUE-2.
Bonnefoy E, Lapostolle F, Leizorovicz A, Steg G, McFadden EP, Dubien PY, Cattan S, Boullenger E, Machecourt J, Lacroute JM, Cassagnes J, Dissait F, Touboul P, of Angioplasty Comparison, Prehospital Thromboysis in Acute Myocardial Infarction study group. Primary angioplasty vs. prehospital FL in acute myocardial infarction: a randomised study.
Steg PG, Bonnefoy E, Chabaud S, Lapostolle F, Dubien PY, Cristofini P, Leizorovicz A, Touboul P, Comparison of Angioplasty Prehospital Thrombolysis In acute Myocardial infarction (CAPTIM) Investigators. Impact of time to treatment on mortality after prehospital FL or primary angioplasty: data from the CAPTIM randomized clinical trial.
Zijlstra F, de Boer MJ, Hoorntje JC, Reiffers S, Reiber JH, Suryapranata H. A comparison of immediate coronary angioplasty with intravenous streptokinase in acute myocardial infarction.
Ribeiro EE, Silva LA, Carneiro R, D'Oliveira LG, Gasquez A, Amino JG, Tavares JR, Petrizzo A, Torossian S, Duprat FR. Randomized trial of direct coronary angioplasty vs. intravenous streptokinase in acute myocardial infarction.
Grinfeld L, Berrocal D, Belardi J, Spinetta A, Matas CR, Oberti P, Doval H, Bazzino O, Cagide A. Fibrinolytics vs. primary angioplasty in acute myocardial infarction (FAP).
Zijlstra F, Beukema WP, van't Hof AW, Liem A, Reiffers S, Hoorntje JC, Suryapranata H, de Boer MJ. Randomized comparison of primary coronary angioplasty with thrombolytic therapy in low risk patients with acute myocardial infarction.
Akhras F, AbuOusa A, Swann G, Duncan H, ChamsiPasha H, Jabbad H. Primary coronary angioplasty or intravenous thrombolysis for patients with acute myocardial infarction? Acute and late follow-up results in a new cardiac unit.
Kedev S, Petrovski B, Kotevski V, Antov S, Sokolov I, Jovanova S. Primary coronary angioplasty vs. intravenous streptokinase in acute myocardial infarction.
de Boer MJ, Ottervanger JP, van't Hof AW, Hoorntje JC, Suryapranata H, Zijlstra F, Zwolle Myocardial Infarction Study Group. Reperfusion therapy in elderly patients with acute myocardial infarction: a randomized comparison of primary angioplasty and thrombolytic therapy.
DeWood MA. Surgical reperfusion vs. rt-PA vs. PTCA as therapy for single vessel LAD anterior myocardial infarction.
Gibbons RJ, Holmes DR, Reeder GS, Bailey KR, Hopfenspirger MR, Gersh BJ. Immediate angioplasty compared with the administration of a thrombolytic agent followed by conservative treatment for myocardial infarction. The Mayo Coronary Care Unit and Catheterization Laboratory Groups.
Grines CL, Browne KF, Marco J, Rothbaum D, Stone GW, O'Keefe J, Overlie P, Donohue B, Chelliah N, Timmis GC. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. The Primary Angioplasty in Myocardial Infarction Study Group.
The Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes (GUSTO IIb) Angioplasty Substudy Investigators. A clinical trial comparing primary coronary angioplasty with tissue plasminogen activator for acute myocardial infarction.
Aoki H, Suzuki T, Shibata M, Takino T, Sato N, Mukaida H, Ohira K, Fukami K, Suzuki T, Hiramori K. A prospective randomized trial of intracoronary t-PA vs. coronary angioplasty in acute myocardial infarction: Japanese Intervention trial in Myocardial Infarction (JIMI).
Morais J, Faria H, Goncalves F, Brandao V, Calisto J, Goncalves L, Andrade C, Castro G, Freitas M, Providencia L. Primary angioplasty in better than front loaded t-PA to preserve left ventricular function after acute anterior myocardial infarction.
Ribichini F, Steffenino G, Dellavalle A, Ferrero V, Vado A, Feola M, Uslenghi E. Comparison of thrombolytic therapy and primary coronary angioplasty with liberal stenting for inferior myocardial infarction with precordial ST-segment depression: immediate and long-term results of a randomized study.
Garcia E, Elizaga J, Perez-Castellano N, Serrano JA, Soriano J, Abeytua M, Botas J, Rubio R, dS Lopez, Lopez-Sendon JL, Delcan JL. Primary angioplasty vs. systemic thrombolysis in anterior myocardial infarction.
Vermeer F, Oude Ophuis AJ, vd Berg EJ, Brunninkhuis LG, Werter CJ, Boehmer AG, Lousberg AH, Dassen WR, Bar FW. Prospective randomised comparison between thrombolysis, rescue PTCA, and primary PTCA in patients with extensive myocardial infarction admitted to a hospital without PTCA facilities: a safety and feasibility study.
Le May MR, Labinaz M, Davies RF, Marquis JF, Laramee LA, O'Brien ER, Williams WL, Beanlands RS, Nichol G, Higginson LA. Stenting vs. thrombolysis in acute myocardial infarction trial (STAT).
Schomig A, Kastrati A, Dirschinger J, Mehilli J, Schricke U, Pache J, Martinoff S, Neumann FJ, Schwaiger M. Coronary stenting plus platelet glycoprotein IIb/IIIa blockade compared with tissue plasminogen activator in acute myocardial infarction. Stent vs. Thrombolysis for Occluded Coronary Arteries in Patients with Acute Myocardial Infarction Study Investigators.
Grines CL, Westerhausen DR Jr, Grines LL, Hanlon JT, Logemann TL, Niemela M, Weaver WD, Graham M, Boura J, O'Neill WW, Balestrini C, Air PAMI Study Group. A randomized trial of transfer for primary angioplasty vs. on-site thrombolysis in patients with high-risk myocardial infarction: the Air Primary Angioplasty in Myocardial Infarction study.
Aversano T, Aversano LT, Passamani E, Knatterud GL, Terrin ML, Williams DO, Forman SA, Atlantic Cardiovascular Patient Outcomes Research Team. Thrombolytic therapy vs. primary percutaneous coronary intervention for myocardial infarction in patients presenting to hospitals without on-site cardiac surgery: a randomized controlled trial.
Kastrati A, Mehilli J, Dirschinger J, Schricke U, Neverve J, Pache J, Martinoff S, Neumann FJ, Nekolla S, Blasini R, Seyfarth M, Schwaiger M, Schomig A. Stent vs. Thrombolysis for Occluded Coronary Arteries in Patients With Acute Myocardial Infarction (STOPAMI). Myocardial salvage after coronary stenting plus abciximab vs. FL plus abciximab in patients with acute myocardial infarction: a randomised trial.
Berlin JA, Laird NM, Sacks HS, Chalmers TC. A comparison of statistical methods for combining event rates from clinical trials.
Snijders TAB, Bosker RJ.
Grines C, Patel A, Zijlstra F, Weaver WD, Granger C, Simes RJ, PCAT Collaborators. Percutaneous transluminal coronary angioplasty. Primary coronary angioplasty compared with intravenous thrombolytic therapy for acute myocardial infarction: six-month follow up and analysis of individual patient data from randomized trials.
Ross AM, Coyne KS, Reiner JS, Greenhouse SW, Fink C, Frey M. A randomized trial comparing primary angioplasty with a strategy of shortacting thrombolysis and immediate planned rescue angioplasty in acute myocardial infarction.
Le May MR, Wells GA, Labinaz M, Davies RF, Turek M, Leddy D, Maloney J, McKibbin T, Quinn B, Beanlands RS, Glover C, Marquis JF, O'Brien ER, Williams WL, Higginson LA. Combined angioplasty and pharmacological intervention vs. thrombolysis alone in acute myocardial infarction (CAPITAL AMI study).
ASSENT-IV Investigators. The assesment of the safety and efficacy of a new treatment strategy for acute myocardial infarction. Presented at the annual scientific sessions of the ESC, Stockholm,
Di Mario C, Bolognese L, Maillard L, Dudek D, Gambarati G, Manari A, Guiducci V, Patrizi G, Rusconi LC, Piovaccari G, Hibon AR, Belpomme V, Indolfi C, Olivari Z, Steffenino G, Zmudka K, Airoldi F, Panzarasa R, Flather M, Steg PG. Combined Abciximab REteplase Stent Study in acute myocardial infarction (CARESS in AMI).
Kent DM, Lau J, Selker HP. Balancing the benefits of primary angioplasty against the benefits of thrombolytic therapy for acute myocardial infarction: the importance of timing.
Nallamothu BK, Bates ER. Percutaneous coronary intervention vs. fibrinolytic therapy in acute myocardial infarction: is timing (almost) everything?
De Luca G, Suryapranata H, Ottervanger JP, Antman EM. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts.
Keeley EC, Boura J, Grines CL. Primary angioplasty or thrombolysis for acute myocardial infarction? [Authors' reply].
Robinson WS. Ecological correlations and the behaviour of individuals.
Every NR, Parsons LS, Hlatky M, Martin JS, Weaver WD. A comparison of thrombolytic therapy with primary coronary angioplasty for acute myocardial infarction. Myocardial Infarction Triage and Intervention Investigators.
Tiefenbrunn AJ, Chandra NC, French WJ, Gore JM, Rogers WJ. Clinical experience with primary percutaneous transluminal coronary angioplasty compared with alteplase (recombinant tissue-type plasminogen activator) in patients with acute myocardial infarction: a report from the Second National Registry of Myocardial Infarction (NRMI-2).
Hasdai D, Behar S, Wallentin L, Danchin N, Gitt AK, Boersma E, Fioretti PM, Simoons ML, Battler A. A prospective survey of the characteristics, treatments and outcomes of patients with acute coronary syndromes in Europe and the Mediterranean basin; the Euro Heart Survey of Acute Coronary Syndromes (Euro Heart Survey ACS).



![Figure 2 Distribution of patients (bars) and median (25th, 75th percentile) time to treatment [FL (▪)] or [PPCI (▪)] according to presentation delay and study treatment.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/eurheartj/27/7/10.1093_eurheartj_ehi810/3/m_ehi81002.jpeg?Expires=1716331053&Signature=SSqiSi5fdGZNgSchNy49vpaGV~5mnd35VTTutNT-ag41JkDDXzxaBtxEa4IRdHyxFX02cqSPtsYCJ~B4oYaL0Z~egs30i23fuDfzGsk47umPnNolnfKgKUY-YtRc6c5oitwdlHflUnIJk08akA73uHlAlbN0hmcqBZPNhPAm~VTp3Dm1RV6T3l~ismiDJyDW0TtXApT~HxjdxmHvEaXqAOalqL6ORj667tg~CRYW1sABoWW0zDjuuyjcQeyiiA9G1jAdrqrj63K4d6w48emdoWQzPsqqikAEW0olZvKbRCjlYai0I7d~v8MxAX-1MrYO3W8ar5rBqxn7mnQ1JsZo6g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)