-
PDF
- Split View
-
Views
-
Cite
Cite
ANNA LARSSON, LENA EDSTRÖM, LENNART SVENSSON, BO SÖDERPALM, JÖRGEN A. ENGEL, VOLUNTARY ETHANOL INTAKE INCREASES EXTRACELLULAR ACETYLCHOLINE LEVELS IN THE VENTRAL TEGMENTAL AREA IN THE RAT, Alcohol and Alcoholism, Volume 40, Issue 5, September/October 2005, Pages 349–358, https://doi.org/10.1093/alcalc/agh180
Close - Share Icon Share
Abstract
Aims: Concurrent use of ethanol and nicotine (tobacco) is often seen in human beings. In previous animal experiments, we have demonstrated that nicotinic acetylcholine receptors, especially α-conotoxin MII and mecamylamine sensitive receptors located in the ventral tegmental area may be involved in the stimulatory, dopamine enhancing, and rewarding effects of ethanol in rodents. Ethanol may exert these effects via direct interaction with nicotinic acetylcholine receptors and/or indirectly via enhancement of extracellular acetylcholine levels in the ventral tegmental area. The present experiments investigated a possible indirect effect of ethanol in stimulating the mesoaccumbal dopamine system. Methods: Neurochemical effects of voluntary ethanol intake on extracellular ventral tegmental acetylcholine and accumbal dopamine levels were measured by means of in vivo microdialysis with a two-probe approach in freely moving rats. Results: Obtained data indicate that voluntary ethanol intake (∼0.7 g/kg/h) leads to an increase of extracellular acetylcholine levels in the ventral tegmental area, and an almost time-locked increase of dopamine levels in the nucleus accumbens. A positive correlation between the ventral tegmental acetylcholine levels and ethanol intake as well as preference was also observed. Conclusion: The present results suggest that voluntary ethanol intake enhances extracellular ventral tegmental acetylcholine that may interact with nicotinic acetylcholine receptors, possibly α-conotoxin MII sensitive receptors, localized in the ventral tegmental area that subsequently may stimulate dopamine overflow in the nucleus accumbens.
(Received 20 April 2005; first review notified 8 May 2005; in revised form 17 May 2005; accepted 19 May 2005)
INTRODUCTION
Several clinical studies have demonstrated that tobacco use and ethanol consumption is highly correlated in human beings (Walton, 1972; Miller and Gold 1998; for review see Bien and Burge, 1990). Smoking prevalence in ethanol-dependent individuals is approximately three times higher compared to the general population (Istavan and Matarazzo, 1984; Sobell et al., 1990), and it has also been demonstrated that an early smoking debut is associated with an increased risk of developing alcoholism (Grant, 1998) and addiction to other drugs later in life (Loimer et al., 1991). Moreover, ethanol consumption is higher in smokers than in non-smokers (Rimm et al., 1995), and the prevalence of ethanol dependence in smokers is 10–14 times higher than among non-smokers (DiFranza and Guerrera, 1990). Results obtained from experimental animal studies demonstrate that chronic ethanol intake induces changes in 3H-nicotine binding in various brain areas (Yoshida et al., 1982), although no significant difference in number of binding sites has also been reported (Nordberg et al., 1985). Findings have also shown that ethanol intake increases in nicotine pre-treated rats (Potthoff et al., 1983; Blomqvist et al., 1996; Clark et al., 2001), providing support for an interaction between ethanol and nicotine also in rodents. Although there may be a common vulnerability involving genetic and/or environmental factors pre-disposing an individual to both nicotine and ethanol abuse, our research group has hypothesized that ethanol and nicotine may share important neurochemical mechanisms of action in stimulating mesolimbic dopamine via direct and/or indirect stimulation of these neurons.
Dopaminergic cellbodies originate in the ventral tegmental area and project to different forebrain structures such as the nucleus accumbens and the prefrontal cortex, forming the mesoaccumbal and mesocortical system, respectively. Several lines of evidence during the last decades indicate that mesoaccumbal dopamine is an important part of the brain reward systems, and that dopamine is involved in mediating some of the acute reinforcing actions of ethanol and other drugs of abuse (Engel, 1977; Engel et al., 1988; Koob, 1992), as well as natural rewards (Kelley and Berridge, 2002). The nucleus accumbens, the terminal region of the mesoaccumbal dopamine system, has been suggested as constituting an important anatomical substrate of the motivational system in the brain; an interface converting motivation to goal directed behaviors (Mogenson et al., 1980; Schultz, 1997).
Regarding the mechanism(s) of action for the stimulatory effects of ethanol on the mesoaccumbal dopamine system, it has been demonstrated that the nicotinic acetylcholine receptors (nAChRs), especially those located in the ventral tegmental area, are involved in mediating the reinforcing and dopamine enhancing effects of ethanol (Ericson et al., 1998; Tizabi et al., 2002). Moreover, nAChRs located in the ventral tegmental area have also been shown to be important in the reinforcing and the dopamine stimulatory effects of nicotine (Corrigall et al., 1992; Nisell et al., 1994). Experiments aimed at defining the subunits of the nAChRs involved in mediating the behavioral and neurochemical effects of ethanol have indicated a role for α-conotoxin MII sensitive receptors (antagonist selective for α3β2*, α6* and β3* nicotinic subunits) but not the α7* and α4β2* subunits of the nAChR (Larsson et al., 2002; Larsson and Engel, 2004; Larsson et al., 2005), whereas the α4β2* and α7* subunits of the nAChR appear to be of importance for the dopamine enhancing effects of nicotine (see e.g. Picciotto et al., 1998; Nomikos et al., 2000; Larsson et al., 2002). Furthermore, in support for a nicotinic–cholinergic ethanol interaction also in human beings, it was recently demonstrated that mecamylamine (an unselective nicotinic antagonist) reduces the subjective stimulant-like effects and reinforcing effects of ethanol (Blomqvist et al., 2002; Chi and de Wit, 2003; but see Rose et al., 2004); and that ethanol potentiated many of the subjective rewarding effects of nicotine (Rose et al., 2004).
The tentative interaction(s) between the ventral tegmental nAChRs and ethanol for the stimulatory effects on the mesoaccumbal dopamine neurons could be brought about via direct and/or indirect effect(s) on these receptors. First, considering a direct effect, it has been demonstrated that ethanol may, at least in vitro (for review see Narahashi et al., 1999), directly interfere with, and stabilize the open channel state of the nAChR (Ei-Fakahany et al., 1983; Wu et al., 1994) from the electric ray Torpedo. Ethanol has also been demonstrated to enhance the electrophysiological response to acetylcholine, indicating that ethanol may also increase the affinity of agonists to the channel activation state (Forman et al., 1989; Nagata et al., 1996; Covernton and Connolly, 1997). Moreover, chronic ethanol exposure has been found to differentially influence mRNA levels of nicotinic receptor subtypes expressed in M10 and SH-SY5Y neuroblastoma cells (Gorbounova et al., 1998). It should however be emphasized that in vitro experiments may be significantly different from results obtained in vivo, where for example the interactions of different excitatory and/or inhibitory neural networks are included.
Second, with regard to an indirect effect of ethanol, evidence has accumulated indicating that cholinergic excitatory inputs to the dopaminergic cellbodies in the ventral tegmental area may constitute an important part of the neuronal circuits mediating natural, as well as drug-rewarded behavior (Yeomans et al., 1985, 1993; Yeomans, 1995; Lança et al., 2000; Rada et al., 2000). Mesencephalic dopamine neurons receive cholinergic input arising from the Ch 5 cell group, localized in the pedunculopontine tegmental nucleus, and the Ch 6 cell group, localized in the laterodorsal tegmental nucleus (Woolf, 1991; Butcher and Woolf, 2003). At least some of these cholinergic neurons are thought to make contact with dopaminergic neurons (Garzon et al., 1999), and in support of this contention, cholinergic agonists locally administered in the ventral tegmental area have been shown to increase extracellular dopamine levels in the nucleus accumbens (Nisell et al., 1994; Blaha et al., 1996; Westerink et al., 1996).
Given the possibility of an indirect effect of ethanol on the ventral tegmental nAChRs, via an enhancement of extracellular acetylcholine levels, we have in the present experiments, by using in vivo microdialysis, investigated the effects on voluntary ethanol intake on extracellular levels of acetylcholine in the ventral tegmental area concomitantly with accumbal dopamine in rats.
MATERIALS AND METHODS
Animals
Male Wistar rats (weighing ∼270 g), purchased from B&K Universal AB (Sollentuna, Sweden) were housed in groups of four in each cage (Macrolon® IV: 550 × 350 × 200 mm) with free access to standard laboratory food (B&K Feeds) and tap water. The animals were allowed to habituate to the animal facilities for at least 1 week upon arrival. A room temperature of 20°C (50% relative humidity) and a reversed 12 h light/dark cycle (lights off at 9:00 a.m. and lights on at 9:00 p.m.) were maintained. The present study was approved by the Ethics committee for animal experiments, Göteborg, Sweden.
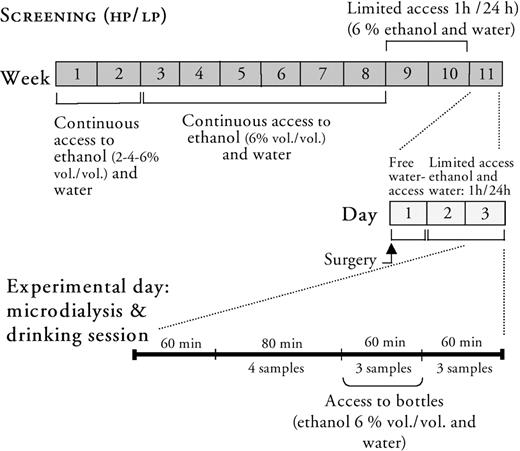
The following procedures for ethanol intake and preference screening of the rats and the experimental design for microdialysis experiments are overviewed in Fig. 1.
Schematic illustration of the experimental procedure used in the present study for screening of high (HP) and low (LP) ethanol preferring rats followed by monitoring extracellular acetylcholine levels in the ventral tegmental area concomitantly with extracellular dopamine levels in the nucleus accumbens in freely moving animals by means of in vivo microdialysis.
Screening procedure for ethanol consumption in rats
The rats (n = 80) were introduced to a two bottle free-choice paradigm with access to tap water and increasing concentrations of ethanol (1 week on 2%, 1 week on 4%) to gradually familiarize them to the ethanol solution. After this period of adjustment to ethanol, the rodents were placed individually in cages (400 × 250 × 200 mm). They were given continuous access to two bottles (300 ml plastic bottles with ball-valve spouts, ALAB, Sweden), each containing either fresh tap water or ethanol solution (6% v/v). The ethanol solution was presented to the left hand side and the water on the right hand side on the same panel of the cage. Food was presented between the bottles. The fluid consumption was measured twice a week by weighing the bottles, and their absolute ethanol intake was expressed as g/kg/day. Ethanol preference was expressed as the proportion of ethanol solution relative to total fluid intake expressed in percent (%). The body weights of the rodents were registered once a week and the bottles were cleaned and filled with fresh beverage at every measurement. Intake of ethanol and water was registered over a 6 week period.
Limited access paradigm
After the continuous access period to ethanol solution and water, the rats were subjected to a limited access paradigm to adapt the animals to the experimental conditions. The animals were given access to ethanol solution (6% v/v) and water during 1 h (11.00 a.m.–12.00 a.m.). Food was offered ad libitum and the fluid restriction paradigm was maintained 2 weeks before surgery. Rats consuming >0.8 g/kg/h (referred to as high ethanol preferring rats, HP) and rats consuming <0.4 g/kg/h (referred to as low ethanol preferring rats, LP) were subjected to in vivo microdialysis experiments. In the present study, the limited access procedure resulted in 5 HP and 8 LP rats out of the initial 80 set to the procedure.
Surgical procedure for microdialysis
To monitor acetylcholine in the ventral tegmental area and dopamine in the nucleus accumbens a dual probe approach was used. After the 1 h drinking session, the rats were anesthetized with isofluran (Isofluran Baxter, Apoteket AB, Sweden; delivered by a Univentor 400 Anaesthesia Unit, Univentor Ltd., Zejtun, Malta) and positioned in a flat skull position in a stereotaxic instrument (David Kopf, Tujunga, CA). Exposure of the skull allowed drilling of two holes for ipsilateral implantation of two probes (nucleus accumbens: +1.9 mm anterior, −1.3 mm lateral; ventral tegmental area: −6.0 mm posterior, −0.6 mm lateral, according to Paxinos and Watson (1998) and two additional holes for stainless steel screws for facilitation of fixation of the probes. I-shaped concentric probes (o.d. 0.32 mm) were hand made with different exposed active surfaces (ventral tegmental area: 1.5 mm and nucleus accumbens: 2 mm). Before insertion of the probes the dura was carefully removed with a thin stainless steel needle. The probes were gently lowered from the brain surface to −7.8 mm (nucleus accumbens) and −8.5 mm (ventral tegmental area) and attached with dental cement (Agntho's AB, Lidingö, Sweden). A plastic collar was provided the animals before awakening and they were allowed to recover in the same cage as prior to surgery for 2 days before the microdialysis experiment. During the first 24 h after surgery the animals had access to water, whereas during the following day the rats were re-adapted to the limited access procedure. The animals did not show any significant change in weight, neither during the limited access procedure nor during the postoperative period before the microdialysis experiment.
Microdialysis sampling
The microdialysis experiments were performed in the same room as the animals were housed. On the experiment day, the dialysis probes were connected to a swivel (Agntho's AB, Lidingö, Sweden) and perfused with Ringer solution at a rate of 1.5 μl/min, delivered by a microperfusion pump (Agntho's AB, Lidingö, Sweden). Acetylcholine was sampled in the ventral tegmental area in the presence of 0.5 μM neostigmine, dissolved in Ringer solution. The acetylcholine esterase inhibitor was used to improve the detection levels of acetylcholine, but needless to say, it cannot be completely excluded that this manipulation might interfere with the experimental conditions. However, the present concentration of neostigmine per se, has not been shown to modify accumbal extracellular dopamine levels (A. Larsson and J. A. Engel, unpublished data). Dopamine was sampled in the nucleus accumbens in Ringer solution only. After habituation to the experimental conditions for 1 h, four samples (with 20 min intervals) were collected from the ventral tegmental area and the nucleus accumbens respectively. Then, the rats were allowed to choose from two bottles, one containing water and the other containing ethanol solution (6% v/v) presented in their cages during 1 h. Six dialysis samples were collected after the rats had had access to the bottles.
Biochemical assay of dopamine
The collected samples from the nucleus accumbens were directly transferred to a cooled (+4°C) autoinjector equipped with a 20 μl loop (CMA/200 refrigerated microsampler CMA Microdialysis AB, Solna, Sweden). The dopamine concentrations were determined by means of high pressure liquid chromatography (HPLC) with electrochemical detection. A pump (P580A, Gyncotec, Germany) delivered the filtered (vacuum filtered: 0.2 μm membrane filter GH Polypro, PALL, USA) mobile phase (5.0 mM citric acid, 10 mM sodium citrate, 0.35 mM 1-decane sulfonic acid, 0.012 mM Na2EDTA and 30% methanol, pH = 5.5) at a rate of 200 μl/min to a reversed-phase column [2.0 × 100 mm, Prodigy 3 μm ODS (3) 100A, Phenomenex, Torrance, CA]. To reduce air content in the mobile phase, a degasser (Degasys Populaire, Japan) was connected prior to the pump. The analyte was oxidized at +0.40 V by using an amperometric detector (Antec Intro, Antec Leyden, The Netherlands) equipped with a VT-03 flow glassy carbon cell (Antec Leyden, The Netherlands). The oxidization was kept under constant temperature (+30°C). Recording and integration of the chromatograms was performed with Dionex Chromeleon software (Dionex, Sunnyvale, CA).
Biochemical assay of acetylcholine
Analysis of the ventral tegmental dialysis samples containing acetylcholine was performed by means of HPLC with electrochemical detection. The dialysates were stored in a −20°C freezer and analyzed within a week. A cooled (+4°C) autoinjector equipped with a 20 μl loop (CMA/200 refrigerated microsampler, CMA Microdialysis AB, Solna, Sweden) was used to inject the dialysis samples. Acetylcholine was separated on a reversed-phase column 2.0 × 150 mm (Eicompak AC-Gel, EICOM, Kyoto, Japan) and subsequently converted to H2O2 by using an immobilized column (Eicompak AC-gel 3.0 × 4.0 mm, Kyoto, Japan) containing acetylcholine esterase and choline oxidase. The enzymatically generated H2O2 was detected (Antec Decade detector, Antec Leyden, The Netherlands) on a glassy carbon electrode (3 mm, VT-03 flow cell, Antec Leyden, The Netherlands) coated with a redox-polymer film containing horseradish peroxidase (MF-2096, BAS, Inc., USA) at a potential of 20.01 V. The mobile phase (pH = 8.2) consisted of 50 mM phosphate buffer containing 1.23 μM sodium 1-decansulfonate and 13.4 mM disodium ethylene-diaminetetraacetic acid, and was delivered by a P580A pump (Gyncotec, Germany) at a flow-rate of 150 μl/min. To reduce air content in the mobile phase, a degasser (Degasys Populaire; Kovalent AB, V. Frölunda, Sweden) was used, coupled prior to the pump. The analysis was kept at a constant temperature (+35°C) and the chromatograms were recorded on and integrated with Dionex Chromeleon software (Dionex, Sunnyvale, CA).
Verification of probe placements
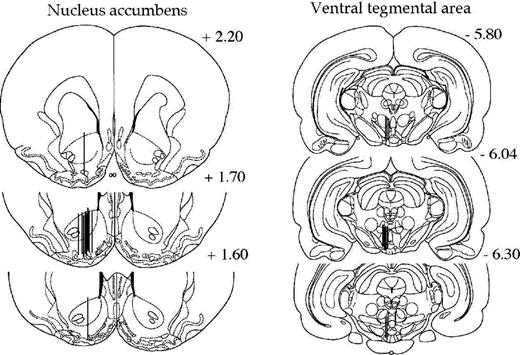
After the experiments, the locations of the two probes (ventral tegmental area and nucleus accumbens) were verified. The rats were decapitated and the probes were perfused with pontamine sky blue 6BX to facilitate probe identification. A vibroslice device (752 M Vibroslice, Campden Instruments Ltd., Loughborough, UK) was used to cut the brains in 50 μm sections. Locations of the probes were identified by gross observation using light microscopy. Figure 2 shows coronal rat brain sections, illustrating microdialysis probe placements within the nucleus accumbens and the ventral tegmental area of all animals in the study [adapted with permission from Paxinos and Watson (1998)].
Coronal rat brain sections showing the probe placements (illustrated by vertical lines) in the nucleus accumbens and the ventral tegmental area of rats used in the present study. The numbers in each brain section indicate millimeters from bregma. Adapted with permission from Paxinos, G. and Watson, C. The Rat Brain in Stereotaxic Coordinates, Figures 10–12 and 43–45. © 1998, Academic Press.
Drugs
Neostigmine bromid (Sigma) was dissolved in Ringer solution: NaCl 140 mM, CaCl2 1.2 mM, KCl 3.0 mM, and MgCl2 1.0 mM and perfused in the ventral tegmental area to facilitate the detection of acetylcholine. Ethanol (VWR International AB, Sweden) was mixed with tap water to desired concentrations (2-4-6% v/v).
Statistics
All data were analyzed by SAS Statview 5.0 computer software (Eurodex Sales AB, Stockholm, Sweden). Acetylcholine and dopamine baseline levels were defined as the averaged levels of the three consecutive 20 min dialysis samples covering the time period −60 to −20 min before start of the drinking session. The microdialysis data were evaluated by a one-way or two-way repeated measurements ANOVA over time. Fishers PLSD test was used following significant two-way repeated measures ANOVA. Changes in dialysate levels over time were analyzed by paired t-tests. Furthermore, linear regression analysis was used to evaluate a possible correlation between extracellular acetylcholine and dopamine levels. Regression analysis was also used to investigate a possible correlation between extracellular acetylcholine levels and ethanol intake, as well as preference. A probability value (P) <0.05 was considered as statistically significant. Error bars in the figures represent standard error of the mean (SEM).
RESULTS
Basal, average baseline levels of the dialysates for extracellular dopamine levels in the nucleus accumbens was 1.04 ± 0.25 nM (all animals included, n = 13), and 5.65 ± 1.08 nM (all animals included, n = 13) for extracellular acetylcholine levels in the ventral tegmental area.
Effects of voluntary ethanol intake on acetylcholine levels (ventral tegmental area) and dopamine levels (nucleus accumbens) in high ethanol preferring rats
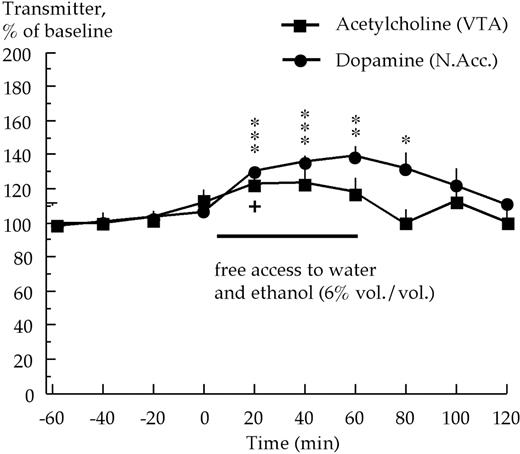
Voluntary ethanol intake (0.7 ± 0.06 g/kg/h, ethanol preference: 40 ± 4.5%) in high ethanol preferring rats (HP, n = 5) caused a significant increase of acetylcholine levels in the ventral tegmental area, Fig. 3 [F(9, 36) = 2.87, P < 0.05, ANOVA: effect of time]. Compared to baseline, ethanol intake induced a statistically significant increase in acetylcholine levels at 20 min (P < 0.05, paired t-test), and was close to being so at 40 min (P = 0.073, paired t-test). Moreover, in the same animals, a significant increase of accumbal dopamine levels was observed [F(9, 36) = 8.46, P < 0.001, ANOVA: effect of time]. Significant increases of extracellular dopamine levels were observed at 20, 40, 60 and 80 min (P < 0.05, paired t-test) compared to baseline levels.
Effects of voluntary ethanol intake on extracellular acetylcholine levels in the ventral tegmental area (VTA) concomitantly with extracellular dopamine levels in the nucleus accumbens (N.Acc.) in high ethanol preferring rats. Acetylcholine and dopamine were monitored by means of in vivo microdialysis (n = 5). ***P < 0.001, **P < 0.01, *P < 0.05 dopamine levels compared to baseline (paired t-test). + P < 0.05, acetylcholine levels at 20 min compared to baseline (paired t-test).
Effects of voluntary ethanol intake on acetylcholine levels (ventral tegmental area) and dopamine levels (nucleus accumbens) in low ethanol preferring rats
Low ethanol preferring rats, with an average ethanol intake of <0.4 g/kg/h (LP, n = 8), were included in the in vivo microdialysis experiments. On the experiment day, some of these rats actually consumed a substantial amount of ethanol, and therefore these rats were divided into two groups: one group of rats (referred to as LP, n = 4) with ethanol intake of <0.4 g/kg/h (average ethanol intake 0.2 ± 0.1 g/kg/h, and ethanol preference: 18.1 ± 8.9%); and the other group of rats (referred to as LP switching to HP rats, n = 4) with ethanol intake of >0.7 g/kg/h (average ethanol intake of 0.8 ± 0.1 g/kg/h, and ethanol preference: 64.2 ± 11.0%).
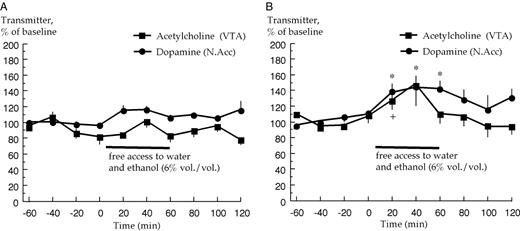
The LP rats did not display a significant increase in either extracellular acetylcholine levels [F(9, 27) = 2.06, P > 0.05, ANOVA: effect of time] or dopamine levels during the drinking session [F(9, 27) = 2.03, P > 0.05, ANOVA: effect of time], Fig. 4A. The LP switching to HP rats, on the other hand, displayed a significant increase of ventral tegmental acetylcholine levels [F(9, 27) = 2.46, P < 0.05, ANOVA: effect of time] as well as an increase of accumbal dopamine levels [F(9, 27) = 5.50, P < 0.001, ANOVA: effect of time] during their drinking session, Fig. 4B. The increase of acetylcholine levels reached statistical significance at 20 min compared to baseline, and the increase of dopamine levels at 20, 40 and 60 min also reached statistical significance (P < 0.05, paired t-test).
Effects of voluntary ethanol intake on extracellular acetylcholine levels in the ventral tegmental area (VTA) and dopamine levels in the nucleus accumbens (N.Acc.) in (A) low ethanol preferring and in (B) low ethanol preferring rats switching to high ethanol preference. Acetylcholine and dopamine were monitored by means of in vivo microdialysis (n = 4, each group). *P < 0.05, dopamine levels at 20, 40 and 60 min compared to baseline (paired t-test). + P < 0.05, acetylcholine levels at 20 min compared to baseline (paired t-test).
A two-way ANOVA of the high ethanol preferring group (HP, n = 5) and the LP switching to HP group (n = 4) with regard to the accumbal dopamine levels revealed a significant change over time [F(7, 9) = 13.0, P < 0.0001, ANOVA: effect of time], and post hoc comparison with Fishers PLSD test did not reveal any difference between the two groups (P < 0.05).
Correlation between acetylcholine levels (ventral tegmental area) and dopamine levels (nucleus accumbens)
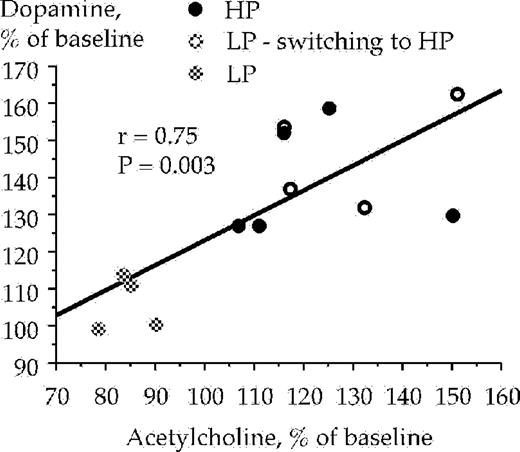
Since it was hypothesized that ethanol may enhance extracellular ventral tegmental acetylcholine levels, and that the enhanced acetylcholine levels may activate mesolimbic dopamine neurons via nAChRs located in the ventral tegmental area, a regression analysis was performed between extracellular acetylcholine and dopamine levels. All animals were included in the analysis (n = 13). A positive correlation was obtained between acetylcholine levels at 20 min and dopamine levels at 60 min [r = 0.75, F(1, 11) = 14.4, P < 0.01, ANOVA], Fig. 5. A similar correlation, almost statistically significant, was also demonstrated between acetylcholine levels at 20 min and dopamine levels at 40 min [r = 0.54, F(1, 11) = 4.65, P = 0.054, ANOVA] but no correlation was obtained between ventral tegmental acetylcholine levels at 20 min and accumbal dopamine levels at 20 min [r = 0.32, F(1, 11) = 1.55, P > 0.05, ANOVA], data not shown.
Correlation between extracellular acetylcholine levels in the ventral tegmental area and dopamine levels in the nucleus accumbens with samples taken between 0–20 (acetylcholine) and 40–60 (dopamine) min respectively, after the animals were presented with a free choice between water and ethanol solution (6% v/v). Acetylcholine and dopamine were monitored by means of in vivo microdialysis in freely moving rats. LP and HP denotes low and high ethanol preferring rats, respectively.
Correlations between acetylcholine levels, ethanol intake and preference
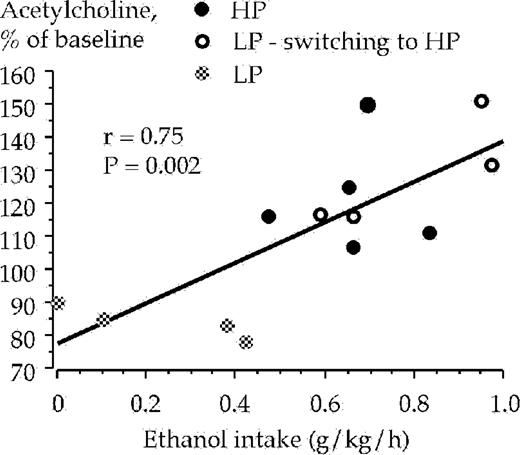
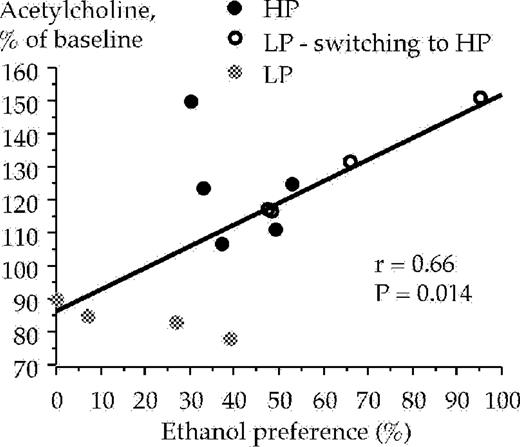
The high and low ethanol preferring animals were subjected to statistical analysis with regard to a possible correlation between extracellular ventral tegmental acetylcholine levels (at 20 min), and their ethanol intake (g/kg/h) and ethanol preference, respectively, during the 1 h drinking session. All animals from the voluntary ethanol intake experiments were included in the analysis (n = 13). A positive correlation was obtained between the ethanol intake and extracellular acetylcholine levels in the ventral tegmental area at 20 min [r = 0.75, F(1,11) = 14.75, P < 0.01, ANOVA], Fig. 6. However, ventral tegmental acetylcholine levels at 40 min did not correlate with ethanol intake [r = 0.50, F(1,11) = 3.72, P = 0.08, ANOVA], data not shown. Positive correlations with regard to ethanol preference and extracellular ventral tegmental acetylcholine levels were demonstrated at timepoint 20 min [r = 0.66, F(1,11) = 8.54, P < 0.05, ANOVA], Fig. 7, and also at timepoint 40 min [r = 0.66, F(1,11) = 8.30, P < 0.05, ANOVA], data not shown.
Correlation between ethanol intake and acetylcholine levels in the ventral tegmental area sampled during the 20 min period after the animals were presented with a free choice between water and ethanol solution (6% v/v). Acetylcholine was monitored by means of in vivo microdialysis in freely moving rats.
Correlation between ethanol preference and acetylcholine levels in the ventral tegmental area sampled during the 20 min period after the animals were presented with a free choice between water and ethanol solution (6% v/v). Acetylcholine was monitored by means of in vivo microdialysis in freely moving rats.
Correlation between dopamine levels, ethanol intake and preference
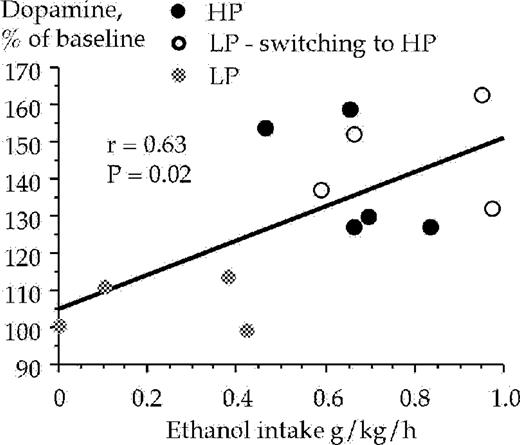
The high and low ethanol preferring animals were also subjected to statistical analysis with regard to a possible correlation between extracellular accumbal dopamine levels and their ethanol intake (g/kg/h) and ethanol preference, during the 1 h drinking session. All animals were included in the analysis (n = 13). A positive correlation was obtained between the ethanol intake and extracellular accumbal dopamine levels at 60 min [r = 0.63, F(1,11) = 7.21, P < 0.05, ANOVA], Fig. 8, and at 40 min [r = 0.68, F(1,11) = 9.39, P < 0.05, ANOVA] but not at 20 min [r = 0.53, F(1,11) = 4.41, P > 0.05, ANOVA]. A positive correlation also occurred regarding ethanol preference and extracellular accumbal dopamine levels at 40 min [r = 0.62, F(1,11) = 7.00, P < 0.05, ANOVA], but not at timepoints 20 min [r = 0.30, F(1,11) = 1.07, P > 0.05, ANOVA] and 60 min [r = 0.30, F(1,11) = 3.80, P = 0.08, ANOVA], data not shown.
Correlation between ethanol intake and dopamine levels in the nucleus accumbens sampled during the 40–60 min period after the animals were presented with a free choice between water and ethanol solution (6% v/v). Dopamine was monitored by means of in vivo microdialysis in freely moving rats.
DISCUSSION
Accumulating evidence indicates that nAChRs, especially those located in the ventral tegmental area, are important in mediating the stimulatory, dopamine enhancing and reinforcing effects of ethanol (Blomqvist et al., 1997; Ericson et al., 1998; Söderpalm et al., 2000; Tizabi et al., 2002). These effects might be exerted via a direct interaction of ethanol with nAChRs in the ventral tegmental area (see Introduction), and/or indirectly by increasing extracellular acetylcholine levels in the ventral tegmental area, that may lead to a stimulation of the nAChRs located also in the ventral tegmental area, and thereby excite the mesoaccumbal dopamine system. In support of the latter hypothesis, we have, in the present experiments, found that voluntary ethanol intake in high ethanol preferring Wistar rats leads to increased extracellular acetylcholine levels in the ventral tegmental area concomitantly, and almost time-locked with an increase of extracellular dopamine in the nucleus accumbens in a two-bottle free choice paradigm. In addition, the extracellular acetylcholine levels in the ventral tegmental area were positively correlated with the ethanol intake and preference, further supporting an association between ethanol and acetylcholine in the ventral tegmental area.
Even though it cannot be excluded that the increases of ventral tegmental acetylcholine and accumbal dopamine levels are parallel phenomena, this appears less likely since these ethanol-induced increases were significantly and positively correlated at least at the sampling intervals 0–20 min (acetylcholine) and 40–60 min (dopamine). This suggests an excitatory relationship between mesopontine acetylcholine and mesoaccumbal dopamine.
Ascending projections of the cholinergic population of the mesencephalic tegmentum are organized topographically such that the dopaminergic cells of the ventral tegmental area are predominantly innervated by the caudal compartment of the pedunculopontine and the laterodorsal tegmental area (Oakman et al., 1995; Butcher and Woolf, 2003). Considering the laterodorsal tegmental area, it has been demonstrated that electrical stimulation of this nucleus leads to an increase of accumbal dopamine overflow, via both nicotinic and muscarinic receptors in the ventral tegmental area (Forster and Blaha, 2000). Findings have shown that cholinergic populations of neurons originating in the pedunculopontine tegmental area are part of the neuronal circuitry that mediates nicotine self-administration (Lança et al., 2000). Furthermore, it has been demonstrated that electrical self-stimulation in the medial forebrain bundle in rats increases extracellular ventral tegmental acetylcholine levels (Nakahara et al., 2001), indicating a possible involvement of mesopontine cholinergic pathways in the brain reward system. However, to the best of our knowledge, our findings are the first to provide direct evidence for a possible role of cholinergic afferents to the ventral tegmental area in dopamine enhancing effects of ethanol. Providing that ethanol may first exert its effects on the cholinergic neurons, one would tentatively expect that the ethanol-induced acetylcholine overflow precludes the enhanced accumbal dopamine overflow. However, the low time resolution of the in vivo microdialysis method does not allow exact determination of the relationship between the acetylcholine and dopamine overflows.
The mechanism by means of which ethanol enhances central acetylcholine remains to be elucidated. The ethanol-induced enhancement of acetylcholine may derive from a direct stimulation of cholinergic afferents to the ventral tegmental area, e.g. laterodorsal and/or pedunculopontine cholinergic neurons. Another possibility is that ethanol may indirectly stimulate the cholinergic afferents for example via interference with neurons connected with cholinergic pathways.
The acetylcholine neurons are widely distributed in the brain and ethanol has been shown to enhance acetylcholine levels in other brain regions. Thus, it was recently demonstrated by means of in vivo microdialysis that systemic (0.5 g/kg i.p.) as well as local administration (50 and 100 mM) of ethanol in the nucleus magnocellularis increases extracellular acetylcholine levels in the prefrontal cortex (Stancampiano et al., 2004). Moreover, increased acetylcholine levels has also been demonstrated by local perfusion with ethanol (50 and 100 mM) in the hippocampus (Henn et al., 1998). In support of an ethanol-induced acetylcholine enhancement, preliminary results obtained in our laboratory indicate that a systemic ethanol injection (0.5 g/kg, i.p.) increases extracellular acetylcholine levels in the ventral tegmental area.
It should be noted that, in addition to the nAChRs, muscarinic acetylcholine receptors have been demonstrated to be present in the ventral tegmental area (Weiner et al., 1990) and might consequently, as the nAChRs, also be activated by the ethanol-induced acetylcholine increase. Stimulation of muscarinic acetylcholine receptors have been shown to increase extracellular dopamine levels in the nucleus accumbens (Gronier et al., 2000) and to play a role in natural reward, for example the ingestion of food (Rada et al., 2000) as well as in brain electrical self-stimulation reward (Yeomans and Baptista, 1997). Consequently, it should be considered that, in addition to the nAChRs, the muscarinic acetylcholine receptors might also contribute to the ethanol-induced stimulatory, rewarding and dopamine enhancing effects.
It might be argued that the elevation of acetylcholine levels in the ventral tegmental area may be due to intake of fluid per se, since it has been shown that acetylcholine levels increase in the ventral tegmental area when water-deprived rats consume water (Rada et al., 2000). For this reason, we have included low ethanol preferring rats to investigate the effects of voluntary fluid intake on ventral tegmental acetylcholine and accumbal dopamine. Our data show that fluid intake by the low ethanol preferring rats with preference for water (natural reinforcer) during the screening period and the experiment day, was not associated with an increase of accumbal dopamine and ventral tegmental acetylcholine. These data suggest that the fluid intake per se was of minor importance for the neurochemical effects obtained. This discrepancy in results, compared to those of Rada and co-workers, may depend on various factors such as different experimental designs; e.g. the rats in the present study were adapted to the fluid limited access paradigm over a 2-week period compared to only 1 day in the study by Rada and co-workers.
Surprisingly, some low ethanol preferring rats actually switched their low-ethanol preference behavior to high ethanol preference, and consumed a substantial amount of ethanol on the experiment day. These rats displayed, in line with the high ethanol-preferring animals, parallel increases in ventral tegmental acetylcholine and accumbal dopamine, further supporting the hypothesis that at least acute ethanol intake can increase extracellular ventral tegmental acetylcholine levels. The reason why some low ethanol preferring rats consumed high amounts of ethanol on the experiment day remains to be clarified. Tentatively, influences of e.g. external sensory stimuli may provide some explanation. Namely, these animals might have experienced the new environmental situation as stressful since they had undergone a surgical procedure and, on the experiment day, were connected to the microdialysis equipment. In this context, it is noteworthy that rats with initially low ethanol preference have been shown to increase their ethanol intake during stress (Bond, 1978; Volpicelli et al., 1990). No statistically significant difference was observed regarding the ethanol-induced accumbal dopamine overflow in the high ethanol preferring rats compared to the low ethanol preferring rats switching to high ethanol preference. This is in agreement with the example of the Alko alcohol and Alko non-alcohol rat-lines, which have not been shown to differ, at least with regard to ethanol-induced dopamine release in the nucleus accumbens (Kiianmaa et al., 1995), but rather have been shown to differ in the basal levels of dopamine and metabolites (Ahtee and Eriksson, 1975). An alternate explanation may be that the high and low ethanol preferring rats may differ at the post synaptic level (see e.g. Engel and Liljequist, 1976).
Even though ethanol was found to increase tegmental acetylcholine levels, a direct stimulatory effect of ethanol on mesoaccumbal dopamine neurons cannot be excluded. A direct effect of ethanol has been demonstrated in acutely dissociated ventral tegmental dopamine neurons isolated from synaptic inputs (Brodie et al., 1990, 1999). In support of these studies, it was recently demonstrated that rats self-administer ethanol in the posterior, but not the anterior part of the ventral tegmental area (Gatto et al., 1994; Rodd-Henricks et al., 2000), indicating that the ventral tegmental area may be an important site for the behavioral and neurochemical effect of ethanol, and also that this region is functionally heterogenic.
The present study shows that ethanol may stimulate cholinergic afferents to the ventral tegmental area, leading to an enhancement of extracellular acetylcholine. This observation provides support for an indirect stimulatory effect of ethanol on the mesoaccumbal dopamine system. Given our previous results that α-conotoxin MII sensitive receptors may be involved in the stimulatory, dopamine enhancing and rewarding effects of ethanol, it is tempting to suggest that the released acetylcholine may interact with these receptors leading to an enhanced accumbal dopamine overflow. The possibility should be considered that ethanol might directly interfere with the nAChR, since it has been shown that ethanol may enhance the electrophysiological response on nAChRs, (see Introduction). Overall, the current findings indicate that modulation of ventral tegmental acetylcholine may serve as a pharmacological target for treatment of ethanol-dependent individuals.
This work was supported by grants from the Swedish Medical Research Council (no 4247 and no 11583) and NIDA 2 R01 10765–04A1, The Swedish Labour Market Insurance (AFA), the Alcohol Research Council of the Swedish Alcohol Retailing Monopoly, the Swedish Match Foundation, Stiftelsen Sigurd and Elsa Golje's Minne, Clas Groschinskys Minnesfond, Wilhelm och Martina Lundgrens Vetenskapsfond, Rådman och Fru Ernst Collianders Stiftelse, The Royal Society of Arts and Science in Göteborg, The Swedish Society for Medical Research and Milan Valverius Stiftelse.
REFERENCES
Ahtee, L. and Eriksson, K. (
Bien, T. H. and Burge, R. (
Blaha, C. D., Allen, L. F., Das, S. et al. (
Blomqvist, O., Ericson, M., Johnson, D. H. et al. (
Blomqvist, O., Ericson, M., Engel, J. A. et al. (
Blomqvist, O., Hernandez-Avila, C. A., Van Kirk, J. et al. (
Bond, N. W. (
Brodie, M. S., Shefner, S. A. and Dunwiddie, T. V. (
Brodie, M. S., Pesold, C. and Appel, S. B. (
Butcher, L. L. and Woolf, N. J. (
Chi, H. and de Wit, H. (
Clark, A., Lindgren, S., Brooks, S. P. et al. (
Corrigall, W. A., Franklin, K. B., Coen, K. M. et al. (
Covernton, P. J. and Connolly, J. G. (
DiFranza, J. R. and Guerrera, M. P. (
Ei-Fakahany, E. F., Miller, E. R., Abbassy, M. A. et al. (
Engel, J. A. (
Engel, J. and Liljequist, S. (
Engel, J. A., Fahlke, C., Hulthe, P. et al. (
Ericson, M., Blomqvist, O., Engel, J. A. et al. (
Ericson, M., Molander, A., Löf, E. et al. (
Forman, S. A., Righi, D. L. and Miller, K. W. (
Forster, G. L. and Blaha, C. D. (
Garzon, M., Vaughan, R. A., Uhl, G. R. et al. (
Gatto, G. J., McBride, W. J., Murphy, J. M. et al. (
Gorbounova, O., Svensson, A.-L., Jönsson, P. et al. (
Grant, B. F. (
Gronier, B., Perry, K. W. and Rasmussen, K. (
Henn, C., Loffelholz, K. and Klein, J. (
Istvan, J. and Matarazzo, J. D. (
Kelley, A. E. and Berridge, K. C. (
Kiianmaa, K., Nurmi, M., Nykanen, et al. (
Koob, G. F. (
Lança, A. J., Adamson, K. L., Coen, K. M. et al. (
Larsson, A., Svensson, L., Söderpalm, B. et al. (
Larsson, A. and Engel, J. A. (
Larsson, A., Jerlhag, E., Svensson, L. et al. (
Loimer, N., Vedovelli, H., Presslich, O. et al. (
Miller, N. S. and Gold, M. S. (
Mogenson, G. J., Jones, D. L. and Yim, C. Y. (
Nagata, K., Aistrup, G. L., Huang, C. S. et al. (
Nakahara, D., Ishida, Y., Nakamura, M. et al. (
Narahashi, T., Aistrup, G. L., Marszalec, W. et al. (
Nisell, M., Nomikos, G. G. and Svensson, T. H. (
Nomikos, G. G., Schilström, B., Hildebrand, B. E. et al. (
Nordberg, A., Wahlström, G. and Eriksson, B. (
Oakman, S. A., Faris, P. L., Kerr, P. E. et al. (
Paxinos, G. and Watson, C. (
Picciotto, M. R., Zoli, M., Rimondini, R. et al. (
Potthoff, A. D., Ellison, G. and Nelson, L. (
Rada, P. V., Mark, G. P., Yeomans, J. J. et al. (
Rimm, E. B., Chan, J., Stampfer, M. J. et al. (
Rodd-Henricks, Z. A., McKinzie, D. L., Crile, R. S. et al. (
Rose, J., Brauer, L., Behm, F. et al. (
Schultz, W. (
Sobell, L. C., Sobell, M. B., Kozlowski, L. T. et al. (
Söderpalm, B., Ericson, M., Olausson, P. et al. (
Stancampiano, R., Carta, M., Cocco, S. et al. (
Tizabi, Y., Copeland, R. L. Jr, Louis, V. A. et al. (
Volpicelli, J. R., Ulm, R. R. and Hopson, N. (
Walton, R. G. (
Weiner, D. M., Levey, A. I. and Brann, M. R. (
Westerink, B. H., Kwint, H. F. and deVries, J. B. (
Woolf, N. J. (
Wu, G., Tonner, P. H. and Miller, K. W. (
Yeomans, J. and Baptista, M. (
Yeomans, J. S. (
Yeomans, J. S., Kofman, O. and McFarlane, V. (
Yeomans, J. S., Mathur, A. and Tampakeras, M. (
Author notes
1Institute of Clinical Neuroscience, Section of Psychiatry, The Sahlgrenska Academy, Göteborg University, SU/Sahlgrenska, SE-413 45 Göteborg, Sweden