Abstract
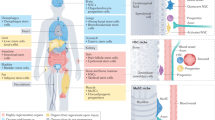
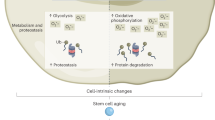
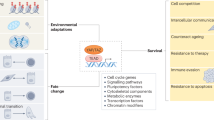
Ageing is accompanied by a progressive decline in stem cell function, resulting in less effective tissue homeostasis and repair. Here we discuss emerging invertebrate models that provide insights into molecular pathways of age-related stem cell dysfunction in mammals, and we present various paradigms of how stem cell functionality changes with age, including impaired self-renewal and aberrant differentiation potential.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kirkwood, T. B. Understanding the odd science of aging. Cell 120, 437–447 (2005).
Gopinath, S. D. & Rando, T. A. Stem cell review series: aging of the skeletal muscle stem cell niche. Aging Cell 7, 590–598 (2008).
Drummond-Barbosa, D. Stem cells, their niches and the systemic environment: an aging network. Genetics 180, 1787–1797 (2008).
Ferraro, F., Celso, C. L. & Scadden, D. Adult stem cells and their niches. Adv. Exp. Med. Biol. 695, 155–168 (2010).
Rando, T. A. Stem cells, ageing and the quest for immortality. Nature 441, 1080–1086 (2006).
Sharpless, N. E. & DePinho, R. A. How stem cells age and why this makes us grow old. Nat Rev. Mol Cell Biol 8, 703–713 (2007).
Pollina, E. A. & Brunet, A. Epigenetic regulation of aging stem cells. Oncogene 10.1038/onc.2011.45 (in the press).
Liu, L. & Rando T. A. Manifestations and mechanisms of stem cell aging. J. Cell Biol. 193, 257–266 (2011).
Kennedy, B. K. The genetics of ageing: insight from genome-wide approaches in invertebrate model organisms. J. Intern. Med. 263, 142–152 (2008).
Kenyon, C. J. The genetics of ageing. Nature 464, 504–512 (2010).
Friedman, D. B. & Johnson, T. E. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics 118, 75–86 (1988).
Kenyon, C., Chang, J., Gensch, E., Rudner, A. & Tabtiang, R. A C. elegans mutant that lives twice as long as wild type. Nature 366, 461–464 (1993).
Kimura, K. D., Tissenbaum, H. A., Liu, Y. & Ruvkun, G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277, 942–946 (1997).
Mair, W. & Dillin, A. Aging and survival: the genetics of life span extension by dietary restriction. Annu. Rev. Biochem. 77, 727–754 (2008).
Kapahi, P. et al. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 11, 453–465 (2010).
Bjedov, I. et al. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 11, 35–46 (2010).
Jasper, H. & Jones, D. L. Metabolic regulation of stem cell behavior and implications for aging. Cell Metab. 12, 561–565 (2010).
Rafalski, V. A. & Brunet, A. Energy metabolism in adult neural stem cell fate. Prog. Neurobiol. 93, 182–203 (2011).
Yilmaz, O. H. et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature 441, 475–482 (2006).
Lee, J. Y. et al. mTOR activation induces tumor suppressors that inhibit leukemogenesis and deplete hematopoietic stem cells after Pten deletion. Cell Stem Cell 7, 593–605 (2010).
Steinkraus, K. A., Kaeberlein, M. & Kennedy, B. K. Replicative aging in yeast: the means to the end. Annu. Rev. Cell Dev. Biol. 24, 29–54 (2008).
Mortimer, R. & Johnston, J. Life span of individual yeast cells. Nature 183, 1751–1752 (1959).
Kennedy, B. K., Austriaco, N. R. Jr, Zhang, J. & Guarente, L. Mutation in the silencing gene SIR4 can delay aging in S. cerevisiae. Cell 80, 485–496 (1995).
Wood, J. G. et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430, 686–689 (2004).
Fabrizio, P. & Longo, V. D. The chronological life span of Saccharomyces cerevisiae. Aging Cell 2, 73–81 (2003).
Aguilaniu, H., Gustafsson, L., Rigoulet, M. & Nystrom, T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science 299, 1751–1753 (2003).
Hsin, H. & Kenyon, C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature 399, 362–366 (1999).
Arantes-Oliveira, N., Apfeld, J., Dillin, A. & Kenyon, C. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science 295, 502–505 (2002).
Greer, E. L. et al. Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans. Nature 466, 383–387 (2010).
Wang, M. C., O'Rourke, E. J. & Ruvkun, G. Fat metabolism links germline stem cells and longevity in C. elegans. Science 322, 957–960 (2008).
Helfand, S. L. & Rogina, B. Molecular genetics of aging in the fly: is this the end of the beginning? Bioessays 25, 134–141 (2003).
Voog, J. & Jones, D. L. Stem cells and the niche: a dynamic duo. Cell Stem Cell 6, 103–115 (2010).
Singh, S. R., Liu, W. & Hou, S. X. The adult Drosophila malpighian tubules are maintained by multipotent stem cells. Cell Stem Cell 1, 191–203 (2007).
Takashima, S., Mkrtchyan, M., Younossi-Hartenstein, A., Merriam, J. R. & Hartenstein, V. The behaviour of Drosophila adult hindgut stem cells is controlled by Wnt and Hh signalling. Nature 454, 651–655 (2008).
Fox, D. T. & Spradling, A. C. The Drosophila hindgut lacks constitutively active adult stem cells but proliferates in response to tissue damage. Cell Stem Cell 5, 290–297 (2009).
Fuller, M. T. & Spradling, A. C. Male and female Drosophila germline stem cells: two versions of immortality. Science 316, 402–404 (2007).
Ohlstein, B. & Spradling, A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439, 470–474 (2006).
Micchelli, C. A. & Perrimon, N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439, 475–479 (2006).
Lin, G., Xu, N. & Xi, R. Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature 455, 1119–1123 (2008).
Renault, V. M. et al. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell 5, 527–539 (2009).
Inomata, K. et al. Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell 137, 1088–1099 (2009).
Paik, J. H. et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell 5, 540–553 (2009).
Wallenfang, M. R., Nayak, R. & DiNardo, S. Dynamics of the male germline stem cell population during aging of Drosophila melanogaster. Aging Cell 5, 297–304 (2006).
Boyle, M., Wong, C., Rocha, M. & Jones, D. L. Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell 1, 470–478 (2007).
Pan, L. et al. Stem cell aging is controlled both intrinsically and extrinsically in the Drosophila ovary. Cell Stem Cell 1, 458–469 (2007).
Cheng, J. et al. Centrosome misorientation reduces stem cell division during ageing. Nature 456, 599–604 (2008).
Margolis, J. & Spradling, A. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development 121, 3797–3807 (1995).
Xie, T. & Spradling, A. C. A niche maintaining germ line stem cells in the Drosophila ovary. Science 290, 328–330 (2000).
Conboy, I. M., Conboy, M. J., Smythe, G. M. & Rando, T. A. Notch-mediated restoration of regenerative potential to aged muscle. Science 302, 1575–1577 (2003).
Brack, A. S. et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 317, 807–810 (2007).
Conboy, I. M. & Rando, T. A. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev. Cell 3, 397–409 (2002).
Conboy, I. M. et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764 (2005).
Carlson, B. M. & Faulkner, J. A. Muscle transplantation between young and old rats: age of host determines recovery. Am. J Physiol. 256, C1262–C1266 (1989).
Conboy, I. M. & Rando, T. A. Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle 4, 407–410 (2005).
LaFever, L. & Drummond-Barbosa, D. Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science 309, 1071–1073 (2005).
Hsu, H. J. & Drummond-Barbosa, D. Insulin levels control female germline stem cell maintenance via the niche in Drosophila. Proc. Natl Acad. Sci. USA 106, 1117–1121 (2009).
Ueishi, S., Shimizu, H. & Inoue, H. Male germline stem cell division and spermatocyte growth require insulin signaling in Drosophila. Cell Struct. Funct. 34, 61–69 (2009).
Wang, L. & Jones, D. L. The effects of aging on stem cell behavior in Drosophila. Exp. Gerontol. 10.1016/j.exger.2010.10.005 (in the press).
Carlson, M. E. et al. Relative roles of TGF-β1 and Wnt in the systemic regulation and aging of satellite cell responses. Aging Cell 8, 676–689 (2009).
Rossi, D. J. et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc. Natl Acad. Sci USA. 102, 9194–9199 (2005).
Beerman, I. et al. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc. Natl Acad. Sci. USA 107, 5465–5470 (2010).
Cho, R. H., Sieburg, H. B. & Muller-Sieburg, C. E. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood 111, 5553–5561 (2008).
Challen, G. A., Boles, N. C., Chambers, S. M. & Goodell, M. A. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-β1. Cell Stem Cell 6, 265–278 (2010).
Choi, Y. J. et al. Age-related upregulation of Drosophila caudal gene via NF-κB in the adult posterior midgut. Biochim. Biophys. Acta 1780, 1093–1100 (2008).
Biteau, B., Hochmuth, C. E. & Jasper, H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell 3, 442–455 (2008).
Park, J. S., Kim, Y. S. & Yoo, M. A. The role of p38b MAPK in age-related modulation of intestinal stem cell proliferation and differentiation in Drosophila. Aging 1, 637–651 (2009).
Jiang, H. et al. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137, 1343–1355 (2009).
Amcheslavsky, A., Jiang, J. & Ip, Y. T. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell 4, 49–61 (2009).
Buchon, N., Broderick, N. A., Chakrabarti, S. & Lemaitre, B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 23, 2333–2344 (2009).
Biteau, B. et al. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS. Genet. 6, e1001159 (2010).
Wessells, R. J., Fitzgerald, E., Cypser, J. R., Tatar, M. & Bodmer, R. Insulin regulation of heart function in aging fruit flies. Nat. Genet. 36, 1275–1281 (2004).
Demontis, F. & Perrimon, N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell 143, 813–825 (2010).
Lin, G., Xu, N. & Xi, R. Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature 455, 1119–1123 (2008).
Campisi, J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120, 513–522 (2005).
Kuro-o M. et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51 (1997).
Kuro-o M. Klotho as a regulator of fibroblast growth factor signaling and phosphate/calcium metabolism. Curr. Opin. Nephrol. Hypertens. 15, 437–441 (2006).
Liu, H. et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 317, 803–806 (2007).
Ayoub, N., Jeyasekharan, A. D., Bernal, J. A. & Venkitaraman, A. R. Paving the way for H2AX phosphorylation: chromatin changes in the DNA damage response. Cell Cycle 8, 1494–1500 (2009).
Rossi, D. J. et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 447, 725–729 (2007).
Charville, G. W. & Rando, T. A. Stem cell ageing and non-random chromosome segregation. Philos. Trans. R. Soc. Lond B Biol. Sci. 366, 85–93 (2011).
Chambers, S. M. et al. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 5, e201 (2007).
Nishino, J., Kim, I., Chada, K. & Morrison, S. J. Hmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16Ink4a and p19Arf expression. Cell 135, 227–239 (2008).
Janzen, V. et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 443, 421–426 (2006).
Krishnamurthy, J. et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature 443, 453–457 (2006).
Signer, R. A., Montecino-Rodriguez, E., Witte, O. N. & Dorshkind, K. Aging and cancer resistance in lymphoid progenitors are linked processes conferred by p16Ink4a and Arf. Genes Dev. 22, 3115–3120 (2008).
Liu, Y. et al. Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell 8, 439–448 (2009).
Blanpain, C., Mohrin, M., Sotiropoulou, P. A. & Passegue, E. DNA-damage response in tissue-specific and cancer stem cells. Cell Stem Cell 8, 16–29 (2011).
Sotiropoulou, P. A. et al. Bcl-2 and accelerated DNA repair mediates resistance of hair follicle bulge stem cells to DNA-damage-induced cell death. Nat. Cell Biol. 12, 572–582 (2010).
von, F. G., Hartmann, D., Song, Z. & Rudolph, K. L. Role of telomere dysfunction in aging and its detection by biomarkers. J. Mol. Med. 87, 1165–1171 (2009).
Flores, I. & Blasco, M. A. The role of telomeres and telomerase in stem cell aging. FEBS Lett. 584, 3826–3830 (2010).
Ju, Z. et al. Telomere dysfunction induces environmental alterations limiting hematopoietic stem cell function and engraftment. Nat. Med. 13, 742–747 (2007).
Song, Z. et al. Alterations of the systemic environment are the primary cause of impaired B and T lymphopoiesis in telomere-dysfunctional mice. Blood 115, 1481–1489 (2010).
Janus, F. et al. The dual role model for p53 in maintaining genomic integrity. Cell Mol. Life Sci. 55, 12–27 (1999).
Tyner, S. D. et al. p53 mutant mice that display early ageing-associated phenotypes. Nature 415, 45–53 (2002).
Maier, B. et al. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 18, 306–319 (2004).
Gannon, H. S., Donehower, L. A., Lyle, S. & Jones, S. N. Mdm2–p53 signaling regulates epidermal stem cell senescence and premature aging phenotypes in mouse skin. Dev. Biol. 353, 1–9 (2011).
Matheu, A. et al. Delayed ageing through damage protection by the Arf/p53 pathway. Nature 448, 375–379 (2007).
Medvedev, Z. A. An attempt at a rational classification of theories of ageing. Biol. Rev. Camb. Philos. Soc. 65, 375–398 (1990).
Kirkwood, T. B. & Holliday, R. The evolution of ageing and longevity. Proc. R. Soc. Lond B Biol. Sci. 205, 531–546 (1979).
Medawar, P. B. An Unsolved Problem of Biology (H. K. Lewis, 1952).
Williams, G. C. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411 (1957).
Blagosklonny, M. V. Revisiting the antagonistic pleiotropy theory of aging: TOR-driven program and quasi-program. Cell Cycle 9, 3151–3156 (2010).
Harrison, D. E. Proliferative capacity of erythropoietic stem cell lines and aging: an overview. Mech. Ageing Dev. 9, 409–426 (1979).
de, H. G., Nijhof, W. & Van, Z. G. Mouse strain-dependent changes in frequency and proliferation of hematopoietic stem cells during aging: correlation between lifespan and cycling activity. Blood 89, 1543–1550 (1997).
Brack, A. S. & Rando, T. A. Intrinsic changes and extrinsic influences of myogenic stem cell function during aging. Stem Cell Rev. 3, 226–237 (2007).
Ryu, B. Y., Orwig, K. E., Oatley, J. M., Avarbock, M. R. & Brinster, R. L. Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells 24, 1505–1511 (2006).
Zhang, X., Ebata, K. T., Robaire, B. & Nagano, M. C. Aging of male germ line stem cells in mice. Biol. Reprod. 74, 119–124 (2006).
Kudlow, B. A., Kennedy, B. K. & Monnat, R. J. Jr. Werner and Hutchinson-Gilford progeria syndromes: mechanistic basis of human progeroid diseases. Nat. Rev. Mol. Cell Biol. 8, 394–404 (2007).
Sahin, E. & DePinho, R. A. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature 464, 520–528 (2010).
Burtner, C. R. & Kennedy, B. K. Progeria syndromes and ageing: what is the connection? Nat. Rev. Mol. Cell Biol. 11, 567–578 (2010).
Nijnik, A. et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 447, 686–690 (2007).
Lee, H. W. et al. Essential role of mouse telomerase in highly proliferative organs. Nature 392, 569–574 (1998).
Rudolph, K. L. et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 96, 701–712 (1999).
Jaskelioff, M. et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 469, 102–106 (2011).
Acknowledgements
We apologize to those colleagues whose work could not be referenced directly owing to space constraints. D.L.J. is funded by the Emerald Foundation, the G. Harold and Leila Y. Mathers Charitable Foundation, the ACS, the California Institute for Regenerative Medicine (CIRM), and the NIH (R01 AG028092). T.A.R. is funded by the NIH (R37 AG23806, R01 AR056849 and an NIH Director's Pioneer Award), the Glenn Foundation for Medical Research, the Department of Veterans Affairs (Merit Review) and the Amertical Federation for Aging Research (“Breakthroughs in Gerontology” (BIG) Award).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Jones, D., Rando, T. Emerging models and paradigms for stem cell ageing. Nat Cell Biol 13, 506–512 (2011). https://doi.org/10.1038/ncb0511-506
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb0511-506
This article is cited by
-
The impact of age-related syndromes on ICU process and outcomes in very old patients
Annals of Intensive Care (2023)
-
Regenerative Engineering of a Limb: From Amputation to Regeneration
Regenerative Engineering and Translational Medicine (2023)
-
RNA sequencing profiles reveal dynamic signaling and glucose metabolic features during bone marrow mesenchymal stem cell senescence
Cell & Bioscience (2022)
-
Aberrant activation of p53/p66Shc-mInsc axis increases asymmetric divisions and attenuates proliferation of aged mammary stem cells
Cell Death & Differentiation (2022)
-
In vivo partial reprogramming alters age-associated molecular changes during physiological aging in mice
Nature Aging (2022)