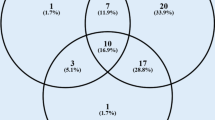
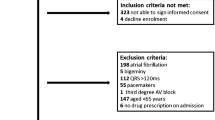
Abstract
Objective
Many elderly patients are routinely exposed to drugs that may prolong the cardiac QT interval and cause Torsades de pointes (TdP). However, predictors of TdP in patients with drug-associated long QT syndrome (LQTS) are not fully understood, especially in the geriatric population. The objective of this study was to identify risk factors of TdP in elderly patients with drug-associated LQTS.
Methods
In this retrospective, case-control study, documented reports of drug-associated LQTS plus TdP (n = 125) and LQTS without TdP (n = 81) in patients ≥65 years of age were retrieved from the French Pharmacovigilance Database over a 10-year period. Available clinical, biological, and drug therapy data were compared in the two groups and logistic regression was performed to identify significant predictors of TdP.
Results
The uncorrected QT interval was significantly longer in patients with TdP than in patients without TdP (577 ± 79 vs. 519 ± 68 ms; p = 0.0001). The number of drugs with a known risk of TdP administered to each patient was not a predictor of arrhythmia, nor was female gender. Logistic regression analysis identified the uncorrected QT interval as the only significant predictor of TdP. The receiver operating characteristic curve analysis was characterized by an area under the curve of 0.77 (95 % confidence interval 0.64–0.88) and a QT cutoff of 550 ms.
Conclusion
The uncorrected QT interval was significantly associated with the probability of TdP in elderly patients with acquired, drug-associated LQTS.

Similar content being viewed by others
References
Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350(10):1013–22. doi:10.1056/NEJMra032426.
Al-Khatib SM, LaPointe NM, Kramer JM, Califf RM. What clinicians should know about the QT interval. JAMA. 2003;289(16):2120–7. doi:10.1001/jama.289.16.2120.
Darpo B. Spectrum of drugs prolonging QT interval and the incidence of torsades de pointes. Eur Heart J Suppl. 2001;3(Suppl K):K70–80.
Poluzzi E, Raschi E, Moretti U, De Ponti F. Drug-induced torsades de pointes: data mining of the public version of the FDA Adverse Event Reporting System (AERS). Pharmacoepidemiol Drug Saf. 2009;18(6):512–8. doi:10.1002/pds.1746.
Molokhia M, Pathak A, Lapeyre-Mestre M, Caturla L, Montastruc JL, McKeigue P. Case ascertainment and estimated incidence of drug-induced long-QT syndrome: study in Southwest France. Br J Clin Pharmacol. 2008;66(3):386–95. doi:10.1111/j.1365-2125.2008.03229.x.
Sauer AJ, Newton-Cheh C. Clinical and genetic determinants of torsade de pointes risk. Circulation. 2012;125(13):1684–94. doi:10.1161/CIRCULATIONAHA.111.080887.
Fermini B, Fossa AA. The impact of drug-induced QT interval prolongation on drug discovery and development. Nat Rev Drug Discov. 2003;2(6):439–47. doi:10.1038/nrd1108.
Haverkamp W, Breithardt G, Camm AJ, Janse MJ, Rosen MR, Antzelevitch C, et al. The potential for QT prolongation and proarrhythmia by non-antiarrhythmic drugs: clinical and regulatory implications. Report on a policy conference of the European Society of Cardiology. Eur Heart J. 2000;21(15):1216–31. doi:10.1053/euhj.2000.2249.
Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart. 2003;89(11):1363–72.
Wolbrette DL. Risk of proarrhythmia with class III antiarrhythmic agents: sex-based differences and other issues. Am J Cardiol. 2003;91(6A):39D–44D.
Zeltser D, Justo D, Halkin A, Prokhorov V, Heller K, Viskin S. Torsade de pointes due to noncardiac drugs: most patients have easily identifiable risk factors. Medicine. 2003;82(4):282–90. doi:10.1097/01.md.0000085057.63483.9b.
Timour Q, Frassati D, Descotes J, Chevalier P, Christe G, Chahine M. Sudden death of cardiac origin and psychotropic drugs. Front Pharmacol. 2012;3:76. doi:10.3389/fphar.2012.00076.
Curtis LH, Ostbye T, Sendersky V, Hutchison S, Allen LaPointe NM, Al-Khatib SM, et al. Prescription of QT-prolonging drugs in a cohort of about 5 million outpatients. Am J Med. 2003;114(2):135–41.
De Ponti F, Poluzzi E, Vaccheri A, Bergman U, Bjerrum L, Ferguson J, et al. Non-antiarrhythmic drugs prolonging the QT interval: considerable use in seven countries. Br J Clin Pharmacol. 2002;54(2):171–7.
Raschi E, Poluzzi E, Zuliani C, Muller A, Goossens H, De Ponti F. Exposure to antibacterial agents with QT liability in 14 European countries: trends over an 8-year period. Br J Clin Pharmacol. 2009;67(1):88–98. doi:10.1111/j.1365-2125.2008.03319.x.
Tisdale JE, Jaynes HA, Kingery JR, Mourad NA, Trujillo TN, Overholser BR, et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6(4):479–87. doi:10.1161/CIRCOUTCOMES.113.000152.
Makkar RR, Fromm BS, Steinman RT, Meissner MD, Lehmann MH. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA. 1993;270(21):2590–7.
Vieweg WV, Wood MA, Fernandez A, Beatty-Brooks M, Hasnain M, Pandurangi AK. Proarrhythmic risk with antipsychotic and antidepressant drugs: implications in the elderly. Drugs Aging. 2009;26(12):997–1012. doi:10.2165/11318880-000000000-00000.
Darbar D, Kimbrough J, Jawaid A, McCray R, Ritchie MD, Roden DM. Persistent atrial fibrillation is associated with reduced risk of torsades de pointes in patients with drug-induced long QT syndrome. J Am Coll Cardiol. 2008;51(8):836–42. doi:10.1016/j.jacc.2007.09.066.
Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 1999;20(2):109–17.
Strnadova C. The assessment of QT/QTc interval prolongation in clinical trials: a regulatory perspective. Drug Inf J. 2005;39:407–33.
US Food and Drug Administration. E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. Guidance for industry 2005. Available from: http://www.fda.gov/RegulatoryInformation/Guidances/ucm129335.htm. Accessed 14 Apr 2014.
Houltz B, Darpo B, Edvardsson N, Blomstrom P, Brachmann J, Crijns HJ, et al. Electrocardiographic and clinical predictors of torsades de pointes induced by almokalant infusion in patients with chronic atrial fibrillation or flutter: a prospective study. Pacing Clin Electrophysiol. 1998;21(5):1044–57.
Buse A. The likelihood ratio, Wald, and Lagrange multiplier tests: an expository note. Am Stat. 1982;36(3):153–7.
Letsas KP, Efremidis M, Kounas SP, Pappas LK, Gavrielatos G, Alexanian IP, et al. Clinical characteristics of patients with drug-induced QT interval prolongation and torsade de pointes: identification of risk factors. Clin Res Cardiol. 2009;98(4):208–12. doi:10.1007/s00392-008-0741-y.
Wang L, Wible BA, Wan X, Ficker E. Cardiac glycosides as novel inhibitors of human ether-a-go-go-related gene channel trafficking. J Pharmacol Exp Ther. 2007;320(2):525–34. doi:10.1124/jpet.106.113043.
Hohnloser SH, Klingenheben T, Singh BN. Amiodarone-associated proarrhythmic effects: a review with special reference to torsade de pointes tachycardia. Ann Intern Med. 1994;121(7):529–35.
Moss AJ, Schwartz PJ, Crampton RS, Tzivoni D, Locati EH, MacCluer J, et al. The long QT syndrome: prospective longitudinal study of 328 families. Circulation. 1991;84(3):1136–44.
Priori SG, Schwartz PJ, Napolitano C, Bloise R, Ronchetti E, Grillo M, et al. Risk stratification in the long-QT syndrome. N Engl J Med. 2003;348(19):1866–74. doi:10.1056/NEJMoa022147.
Ward DE. Prolongation of the QT interval as an indicator of risk of a cardiac event. Eur Heart J. 1988;9(Suppl G):139–44.
Hreiche R, Morissette P, Turgeon J. Drug-induced long QT syndrome in women: review of current evidence and remaining gaps. Gender Med. 2008;5(2):124–35. doi:10.1016/j.genm.2008.05.005.
Rautaharju PM, Zhou SH, Wong S, Calhoun HP, Berenson GS, Prineas R, et al. Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiol. 1992;8(7):690–5.
Funck-Brentano C, Jaillon P. Rate-corrected QT interval: techniques and limitations. Am J Cardiol. 1993;72(6):17B–22B.
Malik M. Problems of heart rate correction in assessment of drug-induced QT interval prolongation. J Cardiovasc Electrophysiol. 2001;12(4):411–20.
Malik M, Farbom P, Batchvarov V, Hnatkova K, Camm AJ. Relation between QT and RR intervals is highly individual among healthy subjects: implications for heart rate correction of the QT interval. Heart. 2002;87(3):220–8.
Davey P. How to correct the QT interval for the effects of heart rate in clinical studies. J Pharmacol Toxicol Methods. 2002;48(1):3–9. doi:10.1016/S1056-8719(03)00008-X.
Joy JP, Coulter CV, Duffull SB, Isbister GK. Prediction of torsade de pointes from the QT interval: analysis of a case series of amisulpride overdoses. Clin Pharmacol Ther. 2011;90(2):243–5. doi:10.1038/clpt.2011.107.
Garnett CE, Zhu H, Malik M, Fossa AA, Zhang J, Badilini F, et al. Methodologies to characterize the QT/corrected QT interval in the presence of drug-induced heart rate changes or other autonomic effects. Am Heart J. 2012;163(6):912–30. doi:10.1016/j.ahj.2012.02.023.
Malik M. The imprecision in heart rate correction may lead to artificial observations of drug induced QT interval changes. Pacing Clin Electrophysiol. 2002;25(2):209–16.
Viskin S. Long QT syndromes and torsade de pointes. Lancet. 1999;354(9190):1625–33. doi:10.1016/S0140-6736(99)02107-8.
Kurita T, Ohe T, Marui N, Aihara N, Takaki H, Kamakura S, et al. Bradycardia-induced abnormal QT prolongation in patients with complete atrioventricular block with torsades de pointes. Am J Cardiol. 1992;69(6):628–33.
Topilski I, Rogowski O, Rosso R, Justo D, Copperman Y, Glikson M, et al. The morphology of the QT interval predicts torsade de pointes during acquired bradyarrhythmias. J Am Coll Cardiol. 2007;49(3):320–8. doi:10.1016/j.jacc.2006.08.058.
de Graaf L, Fabius MA, Diemont WL, van Puijenbroek EP. The Weber-curve pitfall: effects of a forced introduction on reporting rates and reported adverse reaction profiles. Pharm World Sci. 2003;25(6):260–3.
Zabel M, Portnoy S, Franz MR. Electrocardiographic indexes of dispersion of ventricular repolarization: an isolated heart validation study. J Am Coll Cardiol. 1995;25(3):746–52. doi:10.1016/0735-1097(94)00446-W.
Yan GX, Lankipalli RS, Burke JF, Musco S, Kowey PR. Ventricular repolarization components on the electrocardiogram: cellular basis and clinical significance. J Am Coll Cardiol. 2003;42(3):401–9.
Antzelevitch C. Cardiac repolarization : the long and short of it. Europace. 2005;7(Suppl 2):3–9. doi:10.1016/j.eupc.2005.05.010.
Acknowledgements
The French Association of Pharmacovigilance Centres (Association Française des Centres Régionaux de Pharmacovigilance) is gratefully acknowledged for allowing access to the data. The authors had full control of primary data from pharmacovigilance reports.
Conflicts of interest
This work was not supported by any academic, company, or sponsor fund. Sylvain Goutelle, Elodie Sidolle, Michel Ducher, Jacques Caron, Quadiri Timour, Patrice Nony and Aurore Gouraud have no conflicts of interest that are relevant to the content of this study. This work has been presented in part as an oral communication at the 6th meeting of the French Societies of Physiology, Pharmacology and Therapeutics (P2T), Grenoble, 22–24 March 2011, and this presentation has been published as an abstract in Fundamental and Clinical Pharmacology 2011;25(Suppl 1):97 [abstract no. 488].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goutelle, S., Sidolle, E., Ducher, M. et al. Determinants of Torsades de Pointes in Older Patients with Drug-Associated Long QT Syndrome: A Case-Control Study. Drugs Aging 31, 601–609 (2014). https://doi.org/10.1007/s40266-014-0188-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-014-0188-y