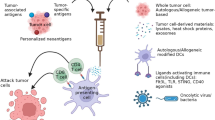
Abstract
Skin cancer, as the most physically accessible malignancy, allows for the greatest variety in treatment innovation. The last 2 decades have seen striking increases in the life expectancies of those diagnosed with malignant melanoma. However, many cases remain in which disease prevails against standard treatment, and those patients rely on continuing ingenuity. Drugs that can be injected directly into patients’ tumors have become increasingly promising, not least for the reduction in side effects observed. Intratumoral therapy encompasses a wide array of agents, from chemotherapeutic drugs to cancer vaccines. While each show some efficacy, those agents which regulate the immune system likely have the greatest potential for preventing disease progression or recurrence. Recent research has highlighted the importance of the presence of cytotoxic T cells and of keeping regulatory T cells in check. Thus, manipulating the tumor microenvironment is a need in skin cancer therapy, which intratumoral delivery can potentially address. In order to find the best approach to each person’s disease, more studies are needed to test intralesional agents in combination with currently approved therapies and with each other.


Similar content being viewed by others
References
Serša G, Štabuc B, Čemažar M, Miklavčič D, Rudolf Z. Electrochemotherapy with cisplatin: clinical experience in malignant melanoma patients. Clin Cancer Res. 2000;6(3):863–7.
Daud AI, Loo K, Pauli ML, et al. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J Clin Investig. 2016;126(9):3447–52. https://doi.org/10.1172/JCI87324.
Wachter EA, Dees C, Harkins J, et al. Functional imaging of photosensitizers using multiphoton microscopy. Multiphoton Microsc Biomed Sci II. 2002;4620:143–148. https://www-spiedigitallibrary-org.ucsf.idm.oclc.org/conference-proceedings-of-spie/4620/0000/Functional-imaging-of-photosensitizers-using-multiphoton-microscopy/10.1117/12.470688.short. Accessed 20 May 2018. https://doi.org/10.1117/12.470688.
Liu H, Weber A, Morse J, et al. T cell mediated immunity after combination therapy with intralesional PV-10 and blockade of the PD-1/PD-L1 pathway in a murine melanoma model. PLoS One. 2018;13(4):e0196033. https://doi.org/10.1371/journal.pone.0196033.
Thompson JF, Hersey P, Wachter E. Chemoablation of metastatic melanoma using intralesional rose bengal. Melanoma Res. 2008;18(6):405–11. https://doi.org/10.1097/CMR.0b013e32831328c7.
Thompson JF, Agarwala SS, Smithers BM, et al. Phase 2 study of intralesional PV-10 in refractory metastatic melanoma. Ann Surg Oncol. 2015;22(7):2135–42. https://doi.org/10.1245/s10434-014-4169-5.
Belehradek M, Domenge C, Luboinski B, Orlowski S, Belehradek J, Mir LM. Electrochemotherapy, a new antitumor treatment. First clinical phase I–II trial. Cancer. 1993;72(12):3694–700. https://doi.org/10.1002/1097-0142(19931215)72:12%3c3694:AID-CNCR2820721222%3e3.0.CO;2-2.
Campana LG, Valpione S, Mocellin S, et al. Electrochemotherapy for disseminated superficial metastases from malignant melanoma. Br J Surg. 2012;99(6):821–30. https://doi.org/10.1002/bjs.8749.
Falk H, Matthiessen LW, Wooler G, Gehl J. Calcium electroporation for treatment of cutaneous metastases; a randomized double-blinded phase II study, comparing the effect of calcium electroporation with electrochemotherapy. Acta Oncol. 2018;57(3):311–9. https://doi.org/10.1080/0284186X.2017.1355109.
Wichtowski M, Murawa D. Electrochemotherapy in the treatment of melanoma. Contemp Oncol (Pozn). 2018;22(1):8–13. https://doi.org/10.5114/wo.2018.74387.
Pike E, Hamidi V, Saeterdal I, Odgaard-Jensen J, Klemp M. Multiple treatment comparison of seven new drugs for patients with advanced malignant melanoma: a systematic review and health economic decision model in a norwegian setting. BMJ Open. 2017;7(8):e014880. https://doi.org/10.1136/bmjopen-2016-014880.
Amgen. Single-arm trial to evaluate the biodistribution and shedding of talimogene laherparepvec. https://clinicaltrials.gov/ct2/show/results/NCT02014441 (2018). Accessed 07 Apr 2019.
BioVex Limited. A study of talimogene laherparepvec in stage IIIc and stage IV malignant melanoma—study results—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/results/NCT00289016. Accessed 07 Apr 2019.
Wang CJ. Intra-lesional nivolumab therapy for limited cutaneous Kaposi sarcoma. https://clinicaltrials.gov/ct2/show/NCT03316274 (2018). Accessed 27 May 2018.
Hofbauer GFI, Baur T, Bonnet M, et al. Clinical phase I intratumoral administration of two recombinant ALVAC canarypox viruses expressing human granulocyte-macrophage colony-stimulating factor or interleukin-2: the transgene determines the composition of the inflammatory infiltrate. Melanoma Res. 2008;18(2):104–11. https://doi.org/10.1097/CMR.0b013e3282f702cf.
Andtbacka RHI, Curti BD, Kaufman H, et al. CALM study: a phase II study of an intratumorally delivered oncolytic immunotherapeutic agent, coxsackievirus A21, in patients with stage IIIc and stage IV malignant melanoma. 2014-06-02T13:15:00Z.
Cassel WA, Murray DR. A ten-year follow-up on stage II malignant melanoma patients treated postsurgically with Newcastle disease virus oncolysate. Med Oncol Tumor Pharmacother. 1992;9(4):169–71.
Zamarin D, Holmgaard RB, Subudhi SK, et al. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med. 2014;6(226):226ra32. https://doi.org/10.1126/scitranslmed.3008095.
Senzer NN, Kaufman HL, Amatruda T, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor–encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. JCO. 2009;27(34):5763–71. https://doi.org/10.1200/JCO.2009.24.3675.
Andtbacka RHI, Agarwala SS, Ollila DW, et al. Cutaneous head and neck melanoma in OPTiM, a randomized phase 3 trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor for the treatment of unresected stage IIIB/IIIC/IV melanoma. Head Neck. 2016;38(12):1752–8. https://doi.org/10.1002/hed.24522.
Andtbacka RHI, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–8. https://doi.org/10.1200/JCO.2014.58.3377.
Ipilimumab with or without talimogene laherparepvec in unresected melanoma. https://clinicaltrials.gov/ct2/show/results/NCT01740297 (2018). Accessed 14 May 2018.
Curti BD, Richards JM, Hallmeyer S, et al. Activity of a novel immunotherapy combination of intralesional coxsackievirus A21 and systemic ipilimumab in advanced melanoma patients previously treated with anti-PD1 blockade therapy. JCO. 2017;35(15_suppl):3014. https://doi.org/10.1200/JCO.2017.35.15_suppl.3014.
Hu JCC, Coffin RS, Davis CJ, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 2006;12(22):6737–47.
BioVex Limited. Efficacy and safety study of talimogene laherparepvec compared to granulocyte macrophage colony stimulating factor (GM-CSF) in melanoma. https://clinicaltrials.gov/ct2/show/results/NCT00769704 (2016). Accessed 06 Apr 2019.
BioVex Limited. An extended use study of safety and efficacy of talimogene laherparepvec in melanoma—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01368276 (2015). Accessed 10 Apr 2019.
Aihara H, Miyazaki J. Gene transfer into muscle by electroporation in vivo. Nat Biotechnol. 1998;16(9):867–70. https://doi.org/10.1038/nbt0998-867.
Till BG, Jensen MC, Wang J, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112(6):2261–71. https://doi.org/10.1182/blood-2007-12-128843.
Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–70. https://doi.org/10.1126/science.1203486.
Sihto H, Böhling T, Kavola H, et al. Tumor infiltrating immune cells and outcome of Merkel cell carcinoma: a population-based study. Clin Cancer Res. 2012;18(10):2872–81. https://doi.org/10.1158/1078-0432.CCR-11-3020.
Daud AI, DeConti RC, Andrews S, et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. JCO. 2008;26(36):5896–903. https://doi.org/10.1200/JCO.2007.15.6794.
Daud A, Algazi AP, Ashworth MT, et al. Systemic antitumor effect and clinical response in a phase 2 trial of intratumoral electroporation of plasmid interleukin-12 in patients with advanced melanoma. JCO. 2014;32(15_suppl):9025. https://doi.org/10.1200/jco.2014.32.15_suppl.9025.
OncoSec Medical Incorporated. IL-12 gene and in vivo electroporation-mediated plasmid DNA vaccine therapy in patients with merkel cell cancer. https://clinicaltrials.gov/ct2/show/NCT01440816 (2019). Accessed 10 Apr 2019.
Canton DA, Shirley S, Wright J, et al. Melanoma treatment with intratumoral electroporation of tavokinogene telseplasmid (pIL-12, tavokinogene telseplasmid). Immunotherapy. 2017;9(16):1309–21. https://doi.org/10.2217/imt-2017-0096.
Bright R, Coventry BJ, Eardley-Harris N, Briggs N. Clinical response rates from interleukin-2 therapy for metastatic melanoma over 30 years’ experience: a meta-analysis of 3312 patients. J Immunother. 2017;40(1):21–30. https://doi.org/10.1097/CJI.0000000000000149.
Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. JCO. 1999;17(7):2105. https://doi.org/10.1200/JCO.1999.17.7.2105.
Shi VY, Tran K, Patel F, et al. 100% complete response rate in patients with cutaneous metastatic melanoma treated with intralesional interleukin (IL)-2, imiquimod, and topical retinoid combination therapy: results of a case series. J Am Acad Dermatol. 2015;73(4):645–54. https://doi.org/10.1016/j.jaad.2015.06.060.
Green DS, Bodman-Smith MD, Dalgleish AG, Fischer MD. Phase I/II study of topical imiquimod and intralesional interleukin-2 in the treatment of accessible metastases in malignant melanoma. Br J Dermatol. 2007;156(2):337–45. https://doi.org/10.1111/j.1365-2133.2006.07664.x.
Bowen RC, Meek S, Williams M, et al. A phase I study of intratumoral injection of ipilimumab and interleukin-2 in patients with unresectable stage III-IV melanoma. JCO. 2015;33(15_suppl):3018. https://doi.org/10.1200/jco.2015.33.15_suppl.3018.
Wahl RU, Braunschweig T, Ghassemi A, Rübben A. Immunotherapy with imiquimod and interferon alfa for metastasized Merkel cell carcinoma. Curr Oncol. 2016;23(2):150. https://doi.org/10.3747/co.23.2878.
Paulson KG, Tegeder A, Willmes C, et al. Downregulation of MHC-I expression is prevalent but reversible in Merkel cell carcinoma. Cancer Immunol Res. 2014;2(11):1071–9. https://doi.org/10.1158/2326-6066.CIR-14-0005.
Chimenti S, Peris K, Di Cristofaro S, Fargnoli MC, Torlone G. Use of recombinant interferon alfa-2b in the treatment of basal cell carcinoma. Dermatology (Basel). 1995;190(3):214–7. https://doi.org/10.1159/000246688.
Bostanci S, Kocyigit P, Alp A, Erdem C, Gurgey E. Treatment of basal cell carcinoma located in the head and neck region with intralesional interferon alpha-2a—evaluation of long-term follow-up results. Clin Drug Investig. 2005;25(10):661–7. https://doi.org/10.2165/00044011-200525100-00005.
Fierlbeck G, Dhoedt B, Stroebel W, Stutte H, Bogenschutz O, Rassner G. Intralesional treatment with recombinant interferon-beta in metastatic melanoma. Hautarzt. 1992;43(1):16–21.
Paul E, Muller I, Renner H, Bodeker RH, Cochran AJ. Treatment of locoregional metastases of malignant melanomas with radiotherapy and intralesional beta-interferon injection. Melanoma Res. 2003;13(6):611–7. https://doi.org/10.1097/01.cmr.0000056285.15046.ff.
Retsas S, Leslie M, Bottomley D. Intralesional tumor necrosis factor combined with interferon gamma in metastatic melanoma. Br Med J. 1989;298(6683):1290–1. https://doi.org/10.1136/bmj.298.6683.1290.
Sparano J, Fisher R, Sunderland M, et al. Randomized phase-III trial of treatment with high-dose interleukin-2 either alone or in combination with interferon alfa-2a in patients with advanced melanoma. J Clin Oncol. 1993;11(10):1969–77. https://doi.org/10.1200/JCO.1993.11.10.1969.
Weide B, Eigentler TK, Pflugfelder A, et al. Intralesional treatment of stage III metastatic melanoma patients with L19–IL2 results in sustained clinical and systemic immunologic responses. Cancer Immunol Res. 2014;2(7):668–78.
Danielli R, Patuzzo R, Di Giacomo AM, et al. Intralesional administration of L19-IL2/L19-TNF in stage III or stage IVM1a melanoma patients: results of a phase II study. Cancer Immunol Immunother. 2015;64(8):999–1009. https://doi.org/10.1007/s00262-015-1704-6.
Samoylenko I, Korotkova OV, Zabotina T, et al. Intralesional anti-PD1 treatment in patients with metastatic melanoma: the pilot study. JCO. 2018;36(5_suppl):188.
Ray A, Williams MA, Meek SM, et al. A phase I study of intratumoral ipilimumab and interleukin-2 in patients with advanced melanoma. Oncotarget. 2016;7(39):64390. https://doi.org/10.18632/oncotarget.10453.
Irenaeus SMM, Nielsen D, Ellmark P, et al. First-in-human study with intratumoral administration of a CD40 agonistic antibody, ADC-1013, in advanced solid malignancies. Int J Cancer. 2019. https://doi.org/10.1002/ijc.32141.
Lardone RD, Chan AA, Lee AF, et al. Mycobacterium bovis bacillus Calmette–Guérin alters melanoma microenvironment favoring antitumor T cell responses and improving M2 macrophage function. Front Immunol. 2017;8:965. https://doi.org/10.3389/fimmu.2017.00965.
Chen XW, Yu TJ, Zhang J, et al. CYP4A in tumor-associated macrophages promotes pre-metastatic niche formation and metastasis. Oncogene. 2017;36(35):5045–57. https://doi.org/10.1038/onc.2017.118.
Goronzy JJ, Fang F, Cavanagh MM, Qi Q, Weyand CM. Naive T cell maintenance and function in human aging. J Immunol. 2015;194(9):4073–80. https://doi.org/10.4049/jimmunol.1500046.
Goding S, Wilson K, Xie Y, et al. Restoring immune function of tumor-specific CD4 + T cells during recurrence of melanoma. J Immunol. 2013;190(9):4899–909. https://doi.org/10.4049/jimmunol.1300271.
Victor CT, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activates non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373. https://doi.org/10.1038/nature14292.
Chew GM, Fujita T, Webb GM, et al. TIGIT marks exhausted T cells, correlates with disease progression, and serves as a target for immune restoration in HIV and SIV infection. PLoS Pathog. 2016;12(1):e1005349. https://doi.org/10.1371/journal.ppat.1005349.
Chauvin J, Pagliano O, Fourcade J, et al. TIGIT and PD-1 impair tumor antigen-specific CD8+T cells in melanoma patients. J Clin Investig. 2015;125(5):2046–58. https://doi.org/10.1172/JCI80445.
Johnston RJ, Comps-Agrar L, Hackney J, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26(6):923–37. https://doi.org/10.1016/j.ccell.2014.10.018.
Inozume T, Yaguchi T, Furuta J, Harada K, Kawakami Y, Shimada S. Melanoma cells control antimelanoma CTL responses via interaction between TIGIT and CD155 in the effector phase. J Investig Dermatol. 2016;136(1):255–63. https://doi.org/10.1038/JID.2015.404.
OncoMed Pharmaceuticals I. A study of OMP-313M32 in subjects with locally advanced or metastatic solid tumors. https://clinicaltrials.gov/ct2/show/NCT03119428 (2018). Accessed 10 Jul 2018.
Kadowaki N, Ho S, Antonenko S, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194(6):863–9.
Wang X, Dong L, Ni H, et al. Combined TLR7/8 and TLR9 ligands potentiate the activity of a Schistosoma japonicum DNA vaccine. PLoS Neglect Trop Dis. 2013;7(4):e2164. https://doi.org/10.1371/journal.pntd.0002164.
Takeuchi O, Takeda K, Horiuchi T, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3(2):196–200. https://doi.org/10.1038/ni758.
Dynavax Technologies Corporation, Merck Sharp & Dohme Corp. A trial of intratumoral injections of SD-101 in combination with pembrolizumab in patients with metastatic melanoma or recurrent or metastatic head and neck squamous cell carcinoma. https://clinicaltrials.gov/ct2/show/NCT02521870 (2018). Accessed 28 Jul 2018.
Checkmate Pharmaceuticals. Clinical study of CMP-001 in combination with pembrolizumab or as a monotherapy. https://clinicaltrials.gov/ct2/show/NCT02680184 (2018). Accessed 28 Jul 2018.
Idera Pharmaceuticals I. A study to assess the safety and efficacy of intratumoral IMO-2125 in combination with ipilimumab or pembrolizumab in patients with metastatic melanoma. https://clinicaltrials.gov/ct2/show/NCT02644967 (2018). Accessed 29 Jul 2018.
Milhem M, Gonzales R, Medina T, et al. Intratumoral toll-like receptor 9 (TLR9) agonist, CMP-001, in combination with pembrolizumab can reverse resistance to PD-1 inhibition in a phase Ib trial in subjects with advanced melanoma. AACR Annual Meeting 2018. 2018. http://www.abstractsonline.com/pp8/#!/4562/presentation/11133. Accessed 13 May 2018.
Mosier DE. A requirement for two cell types for antibody formation in vitro. Science. 1967;158(3808):1573–5.
Belz GT, Behrens GMN, Smith CM, et al. The CD8alpha(+) dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J Exp Med. 2002;196(8):1099–104.
Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8(12):935–47. https://doi.org/10.1038/nri2455.
Bogunovic M, Ginhoux F, Helft J, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31(3):513–25. https://doi.org/10.1016/j.immuni.2009.08.010.
Ginhoux F, Liu K, Helft J, et al. The origin and development of nonlymphoid tissue CD103 + DCs. J Exp Med. 2009;206(13):3115–30. https://doi.org/10.1084/jem.20091756.
Steptoe RJ, Patel RK, Subbotin VM, Thomson AW. Comparative analysis of dendritic cell density and total number in commonly transplanted organs: morphometric estimation in normal mice. Transpl Immunol. 2000;8(1):49–56. https://doi.org/10.1016/S0966-3274(00)00010-1.
Tumeh PC, Hellmann MD, Hamid O, et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res. 2017;5(5):417–24. https://doi.org/10.1158/2326-6066.CIR-16-0325.
Scharcanski J, Celebi ME. Computer vision techniques for the diagnosis of skin cancer. 1st ed. Berlin: Springer; 2014. http://www.springer.com/us/book/9783642396076 (2014). Accessed 13 May 2018.
Irvine DJ, Hanson MC, Rakhra K, Tokatlian T. Synthetic nanoparticles for vaccines and immunotherapy. Chem Rev. 2015;115(19):11109.
Cheng Y, Chang Y, Feng Y, et al. Simulated sunlight-mediated photodynamic therapy for melanoma skin cancer by titanium-dioxide-nanoparticle-gold-nanocluster-graphene heterogeneous nanocomposites. Small. 2017. https://doi.org/10.1002/smll.201603935.
Carrasco E, del Rosal B, Sanz-Rodríguez F, et al. Intratumoral thermal reading during photo-thermal therapy by multifunctional fluorescent nanoparticles. Adv Funct Mater. 2015;25(4):615–26. https://doi.org/10.1002/adfm.201403653.
Walther W, Siegel R, Kobelt D, et al. Novel jet-injection technology for nonviral intratumoral gene transfer in patients with melanoma and breast cancer. Clin Cancer Res. 2008;14(22):7545–53. https://doi.org/10.1158/1078-0432.CCR-08-0412.
Langer R, Prausnitz MR. Transdermal drug delivery. Nat Biotechnol. 2008;26(11):1261–8. https://doi.org/10.1038/nbt.1504.
Harvey A, Kaestner S, Sutter D, Harvey N, Mikszta J, Pettis R. Microneedle-based intradermal delivery enables rapid lymphatic uptake and distribution of protein drugs. Pharm Res. 2011;28(1):107–16. https://doi.org/10.1007/s11095-010-0123-9.
Labala S, Jose A, Chawla SR, et al. Effective melanoma cancer suppression by iontophoretic co-delivery of STAT3 siRNA and imatinib using gold nanoparticles. Int J Pharm. 2017;525(2):407–17. https://doi.org/10.1016/j.ijpharm.2017.03.087.
Kim SE, Salvi SM. Immunoreduction of ocular surface tumours with intralesional interferon alpha-2a. Eye (London, England). 2018;32(2):460–2. https://doi.org/10.1038/eye.2017.196.
Nantes University Hospital, MEDA Pharma GmbH & Co. KG. Relevance of imiquimod as neo-adjuvant treatment to reduce excision size and the risk of intralesional excision in lentigo malignant of the face. https://clinicaltrials.gov/ct2/show/NCT01720407 (2017). Accessed 27 May 2018.
Andtbacka RHI, Dummer R, Gyorki DE, et al. Interim analysis of a randomized, open-label phase 2 study of talimogene laherparepvec (T-VEC) neoadjuvant treatment (neotx) plus surgery (surgx) vs surgx for resectable stage IIIB-IVM1a melanoma (MEL). J Clin Oncol. 2018;36:9508.
Stravodimou A, Tzelepi V, Papadaki H, et al. Evaluation of T-lymphocyte subpopulations in actinic keratosis, in situ and invasive squamous cell carcinoma of the skin. J Cutan Pathol. 2018;45(5):337–47. https://doi.org/10.1111/cup.13123.
Fu J, Kanne DB, Leong M, et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med. 2015. https://doi.org/10.1126/scitranslmed.aaa4306.
Khammari A, Nguyen J, Saint-Jean M, et al. Adoptive T cell therapy combined with intralesional administrations of TG1042 (adenovirus expressing interferon-γ) in metastatic melanoma patients. Cancer Immunol Immunother. 2015;64(7):805–15. https://doi.org/10.1007/s00262-015-1691-7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of interest
Ms. Oglesby declares that she has no conflicts of interest that might be relevant to the contents of this manuscript. Dr. Algazi reports Grants from Merck, Acerta, Medimmune, AstraZeneca, Genentech, Dynavax, Tessa, and OncoSec; personal fees and non-financial support from Valitor, and OncoSec; personal fees from Array; and Grants from Incyte, Idera, Plexxicon, Checkmate, Regeneron, Novartis, Amgen, and BMS outside the submitted work. Dr. Daud reports Grants from Merck and BMS; Grants and personal fees from Incyte; Grants from Pfizer, Genentech, OncoSec, Takara, and Checkmate; Grants and personal fees from Novartis; and Grants from Exelixis outside the submitted work.
Rights and permissions
About this article
Cite this article
Oglesby, A., Algazi, A.P. & Daud, A.I. Intratumoral and Combination Therapy in Melanoma and Other Skin Cancers. Am J Clin Dermatol 20, 781–796 (2019). https://doi.org/10.1007/s40257-019-00452-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-019-00452-8