Abstract
Objective
To study the neuroprotective mechanism of minocycline against vascular cognitive impairment after cerebral ischemia.
Methods
The rat model with vascular cognitive impairment was established by permanent bilateral common carotid artery occlusion (BCCAO). The observing time-points were determined at 4, 8 and 16 weeks after BCCAO. Animals were randomly divided into sham-operated group (n = 6), model group (subdivided into 3 groups: 4 weeks after BCCAO, n = 6; 8 weeks after BCCAO, n = 6; and 16 weeks after BCCAO, n = 6), and minocycline group (subdivided into 3 groups: 4 weeks after BCCAO, n = 6; 8 weeks after BCCAO, n = 6; and 16 weeks after BCCAO, n = 6). Minocycline was administered by douche via stomach after BCCAO until sacrifice. Glial fibrillary acidic protein (GFAP) was examined by Western blotting and immunohistochemistry. Levels of cyclooxygenase-2 (COX-2) and nuclear factor-kappaB (NF-κB) were measured by immunohistochemistry. IL-1β and TNF-α levels were tested with ELISA method.
Results
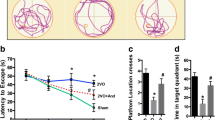
Levels of GFAP, COX- 2, NF-κB, IL-1β and TNF-α were all up-regulated after permanent BCCAO, which could be significantly inhibited by minocycline.
Conclusion
Minocycline could ameliorate the inflammation and oxidative stress in the hippocampus of the vascular cognitive impairment rat model.
摘要
目的
观察美满霉素(minocycline)对血管性认知功能损伤大鼠海马组织GFAP、 COX-2、 NF-κB、 IL-1β 和 TNF-α表达的影响, 探讨美满霉素对血管性认知功能损伤脑保护作用的机制。
方法
Wistar大鼠随机分为假手术组 (S组)、血管性认知功能损伤模型组(M组)和美满霉素治疗组(MT组)。 免疫组织化学法检测大鼠海马组织COX-2和 NF-κB的表达, 蛋白质印迹和免疫组织化学法检测大鼠海马组织GFAP的表达, ELISA法检测大鼠海马组织IL-1β和 TNF-α 的表达。
结果
MT 组GFAP、COX-2、 NF-κB、 IL-1β 和TNF-α 表达较M组均降低(P<0.01); MT 和M组 GFAP、COX-2、 NF-κB、 IL-1β 和TNF-α 表达均显著高于S 组(P<0.01)。
结论
美满霉素能降低血管性认知功能损伤大鼠海马组织中GFAP、 COX-2、 NF-κB、 IL-1β 和TNF-α 的表达, 抑制血管性认知功能损伤大鼠海马星型胶 质细胞活化和神经炎症, 发挥脑保护作用。
Similar content being viewed by others
References
Kumaran D, Udayabanu M, Kumar M, Aneja R, Katyal A. Involvement of angiotensin converting enzyme in cerebral hypoperfusion induced anterograde memory impairment and cholinergic dysfunction in rats. Neuroscience 2008, 155(3): 626–639.
Lee JH, Park SY, Shin HK, Kim CD, Lee WS, Hong KW. Protective effects of cilostazol against transient focal cerebral ischemia and chronic cerebral hypoperfusion injury. CNS Neurosci Ther 2008, 14(2): 143–152.
He Z, Huang L, Wu Y, Wang J, Wang H, Guo L. DDPH: improving cognitive deficits beyond its alpha 1-adrenoceptor antagonism in chronic cerebral hypoperfused rats. Eur J Pharmacol 2008, 588(2–3): 178–188.
Zadori D, Datki Z, Penke B. The role of chronic brain hypoperfusion in the pathogenesis of Alzheimer’s disease-facts and hypotheses. Ideggyogy Sz 2007, 660(11-12): 428–437.
Zheng P, Zhang J, Liu H, Xu X, Zhang X. Angelica injection reduces cognitive impairment during chronic cerebral hypoperfusion through brain-derived neurotrophic factor and nerve growth factor. Curr Neurovasc Res 2008, 5(1): 13–20.
Yrjänheikki J, Tikka T, Keinänen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A 1999, 96(23): 13496–13500.
Alvaro-Gonzalez LC, Freijo-Guerrero MM, Sadaba-Garay F. Inflammatory mechanisms, arteriosclerosis and ischemic stroke: clinical data and perspectives. Rev Neurol 2002, 35(5): 452–462.
Hamel E, Nicolakakis N, Aboulkassim T, Ongali B, Tong XK. Oxidative stress and cerebrovascular dysfunction in mouse models of Alzheimer’s disease. Exp Physiol 2008, 93(1): 116–120.
Gackowski D, Rozalski R, Siomek A, Dziaman T, Nicpon K, Klimarczyk M, et al. Oxidative stress and oxidative DNA damage is characteristic for mixed Alzheimer disease/vascular dementia. J Neurol Sci 2008, 266(1–2): 57–62.
Price DL, Sisodia SS, Gandy SE. Amyloid beta amyloidosis in Alzheimer’s disease. Curr Opin Neurol 1995, 8(4): 268–274.
Juravleva E, Barbakadze T, Mikeladze D, Kekelidze T. Creatine enhances survival of glutamate-treated neuronal/glial cells, modulates Ras/NF-kappaB signaling, and increases the generation of reactive oxygen species. J Neurosci Res 2005, 79(1–2): 224–230.
Rosenberger J, Petrovics G, Buzas B. Oxidative stress induces proorphanin FQ and proenkephalin gene expression in astrocytes through p38- and ERK-MAP kinases and NF-kappaB. J Neurochem 2001, 79: 35–44.
Swanson RA, Ying W, Kauppinen TM. Astrocyte influences on ischemic neuronal death. Curr Mol Med 2004, 4: 193–205.
Gabriel C, Justicia C, Camins A, Planas AM. Activation of nuclear factor-kappaB in the rat brain after transient focal ischemia. Brain Res Mol Brain Res 1999, 65: 61–69.
He XL, Wang YH, Gao M, Li XX, Zhang TT, Du GH. Baicalein protects rat brain mitochondria against chronic cerebral hypoperfusion-induced oxidative damage. Brain Res 2009, 1249: 212–221.
Kasparová S, Brezová V, Valko M, Horecký J, Mlynárik V, Liptaj T, et al. Study of the oxidative stress in a rat model of chronic brain hypoperfusion. Neurochem Int 2005, 46: 601–611.
Imre SG, Fekete I, Farkas T. Increased proportion of docosahexanoic acid and high lipid peroxidation capacity in erythrocytes of stroke patients. Stroke 1994, 25: 2416–2420.
Andersson A, Lindgren A, Hultberg B. Effect of thiol oxidation and thiol export from erythrocytes on determination of redox status of homocysteine and other thiols in plasma from healthy subjects and patients with cerebral infarction. Clin Chem 1995, 41: 361–366
Endoh M, Maiese K, Wagner J. Expression of the inducible form of nitric oxide synthase by reactive astrocytes after transient global ischemia. Brain Res 1994, 651: 92–100.
Acarin L, Peluffo H, Barbeito L, Castellano B, González B. Astroglial nitration after postnatal excitotoxic damage: correlation with nitric oxide sources, cytoskeletal, apoptotic and antioxidant proteins. J Neurotrauma 2005, 22(1): 189–200.
Morimoto N, Shimazawa M, Yamashima T, Hara H. Minocycline inhibits oxidative stress and decreases in vitro and in vivo ischemic neuronal damage. Brain Res 2005, 1044: 8–15.
Cai ZY, Yan Y, Sun SQ, Zhang J, Huang LG, Yan N, et al. Minocycline attenuates cognitive impairment and restrains oxidative stress in the hippocampus of rats with chronic cerebral hypoperfusion. Neurosci Bull 2008, 24(5): 305–313.
Yrjänheikki J, Tikka T, Keinänen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A 1999, 96: 13496–13500.
Kántor O, Schmitz C, Feiser J, Brasnjevic I, Korr H, Busto R, et al. Moderate loss of cerebellar Purkinje cells after chronic bilateral common carotid artery occlusion in rats. Acta Neuropathol 2007, 113(5): 549–558.
Stirling DP, Khodarahmi K, Liu J, McPhail LT, McBride CB, Steeves JD, et al. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci 2004, 24: 2182–2190.
Hewlett KA, Corbett D. Delayed minocycline treatment reduces long-term functional deficits and histological injury in a rodent model of focal ischemia. Neuroscience 2006, 141: 27–33.
Guo M, Cox B, Mahale S, Davis W, Carranza A, Hayes K, et al. Pre-ischemic exercise reduces matrix metalloproteinase-9 expression and ameliorates blood-brain barrier dysfunction in stroke. Neuroscience 2008, 151: 340–351.
Mendes O, Kim HT, Stoica G. Expression of MMP2, MMP9 and MMP3 in breast cancer brain metastasis in a rat model. Clin Exp Metastasis 2005, 22: 237–246.
Sandya S, Sudhakaran PR. Effect of glycosaminoglycans on matrix metalloproteinases in type II collagen-induced experimental arthritis. Exp Biol Med (Maywood) 2007, 232: 629–637.
Weng YC, Kriz J. Differential neuroprotective effects of a minocycline-based drug cocktail in transient and permanent focal cerebral ischemia. Exp Neurol 2007, 204: 433–442.
Rosenberg GA, Estrada EY, Mobashery S. Effect of synthetic matrix metalloproteinase inhibitors on lipopolysaccharide-induced blood-brain barrier opening in rodents: Differences in response based on strains and solvents. Brain Res 2007, 1133: 186–192.
Xu L, Fagan SC, Waller JL, Edwards D, Borlongan CV, Zheng J, et al. Low dose intravenous minocycline is neuroprotective after middle cerebral artery occlusion-reperfusion in rats. BMC Neurol 2004, 4: 7.
Pattison LR, Kotter MR, Fraga D, Bonelli RM. Apoptotic cascades as possible targets for inhibiting cell death in Huntington’s disease. J Neurol 2006, 253: 1137–1142.
Choi Y, Kim HS, Shin KY, Kim EM, Kim M, Kim HS, et al. Minocycline attenuates neuronal cell death and improves cognitive impairment in Alzheimer’s Disease models. Neurosci Res 2007, 58(S1): S81.
Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature 2002, 417: 74–78.
Wang X, Zhu S, Drozda M, Zhang W, Stavrovskaya IG, Cattaneo E, et al. Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington’s disease. Proc Natl Acad Sci U S A 2003, 100: 10483–10487.
Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, et al. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci 2002, 22: 1763–1771.
Chu LS, Fang SH, Zhou Y, Yu GL, Wang ML, Zhang WP, et al. Minocycline inhibits 5-lipoxygenase activation and brain inflammation after focal cerebral ischemia in rats. Acta Pharmacol Sin 2007, 28: 763–772.
Liu Z, Fan Y, Won SJ, Neumann M, Hu D, Zhou L, et al. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke 2007, 38: 146–152.
Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG. Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke 2006, 37: 1087–1093.
Wang JY, Wen LL, Huang YN, Chen YT, Ku MC. Dual effects of antioxidants in neurodegeneration: direct neuroprotection against oxidative stress and indirect protection via suppression of gliamediated inflammation. Curr Pharm Des 2006, 12(27): 3521–3533.
Liu X, Sullivan KA, Madl JE, Legare M, Tjalkens RB. Manganese-induced neurotoxicity: the role of astroglial-derived nitric oxide in striatal interneuron degeneration. Toxicol Sci 2006, 91(2): 521–531.
Buskila Y, Farkash S, Hershfinkel M, Amitai Y. Rapid and reactive nitric oxide production by astrocytes in mouse neocortical slices. Glia 2005, 52(3): 169–176.
Jing R, Wilhelmsson U, Goodwill W, Li L, Pan Y, Pekny M, et al. Synemin is expressed in reactive astrocytes in neurotrauma and interacts differentially with vimentin and GFAP intermediate filament networks. J Cell Sci 2007, 120(Pt 7): 1267–1277.
Van Beek J, Chan P, Bernaudin M, Petit E, MacKenzie ET, Fontaine M. Glial responses, clusterin, and complement in permanent focal cerebral ischemia in the mouse. Glia 2000, 31(1): 39–50.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, ZY., Yan, Y. & Chen, R. Minocycline reduces astrocytic reactivation and neuroinflammation in the hippocampus of a vascular cognitive impairment rat model. Neurosci. Bull. 26, 28–36 (2010). https://doi.org/10.1007/s12264-010-0818-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-010-0818-2