Abstract
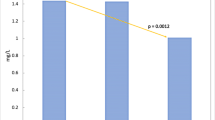
The medical literature on the effect of electronic control devices (ECD) on muscle injury is sparse. In this paper, we examine pooled data from five human studies that used creatine kinase (CK) as a marker for muscle injury. CK was measured in five separate studies involving four TASER ECDs with different exposure durations and number of circuits or contact points. Device type, exposure duration, number of circuits or contact points, and CK values at baseline and 24 h after exposure were pooled from these previous studies. Data were analyzed to determine the correlation of CK to duration of exposure, number of contact points, and distance between the probes. The pooled results contained 163 subjects. Seven were withdrawn due to incomplete data, leaving 156 subjects for analysis (median age 36, range 19–67, 93.6% male). 121 (77.6%) subjects had 2 contacts points, 10 (6.4%) had 3 contact points, 18 (11.5%) had 4 contact points, and 7 (4.5%) had 6 contact points. 81 (51.9%) subjects had a 5-s exposure, 64 (41.0%) a 10-s exposure, and, 11 (7.1%) a 30-s exposure. Median baseline CK (145 U/l, IQR 104–217, range 12–1956) did not differ between groups (P = 0.213 for number of contact points, 0.124 for duration). For the number of contacts, the median change in CK for 2 points of contact was 32 (IQR −1 to 1513, range −205 to 1821), for 3 was 1456 (IQR 634–1868, range 101–25452), for 4 was 887 (IQR 285–7481, range −1054 to 7481), and for 6 was 846 (IQR 57–1149, range −8 to 2309), (P < 0.001). For duration, the median change in CK for 5 s was 26.5 (IQR −8 to 109, range −1054 to 2309), for 10 s was 303 (IQR 34.5–1073, range −205 to 25452), and for 30 s was 47 (IQR 23–82, range −140 to 364), (P < 0.001). There was a relationship between the number of points of contact and the change in CK (P < 0.001) but not a relationship between the duration and the change in CK (P = 0.496). The median spread between the probe pairs for our pooled data was 40 cm, with a range from 18 to 70 cm (n = 76). The correlation between the change in CK and spread between the probe pairs was 0.16 at baseline (P = 0.18), and 0.24 at 24 h (P = 0.04) by Spearman’s rank correlation. ECD exposure can cause a modest increase in CK. Although we cannot draw conclusions about the individual devices included in this analysis, our findings indicated that multiple contact points or exposures may result in a larger increase in CK, but the duration of the exposure does not appear to have a significant effect on CK. There is a correlation between the distance between the probes and the change in CK.

Similar content being viewed by others
References
Reilly J, Diamant A, Comeaux J. Dosimetry considerations for electrical stun devices. Phys Med Biol. 2009;54:1319–35.
Despa F, Basati S, Zhang Z, D’Andrea J, Reilly J, Bodnar E, Lee R. Electromuscular incapacitation results from stimulation of spinal reflexes. Bioelectromagnetics. 2009;30:411–21.
Sun H, Webster J. Estimating neuromuscular stimulation within the human torso with TASER stimulus. Phys Med Biol. 2007;52:6401–11.
Ho JD, Miner JR, Lakireddy DR, et al. Cardiovascular and physiologic effects of conducted electrical weapon discharge in resting adults. Acad Emerg Med. 2006;13:589–95.
Dawes D, Ho J, Reardon R, Sweeney J, Miner J. The physiologic effects of multiple simultaneous electronic control device discharges. West J Emerg Med. 2010;11:49–56.
Brancaccio P, Maffulli N, Buonauro R, Limongelli F. Serum enzyme monitoring in sports medicine. Clin Sports Med. 2008;27:1–18.
Jauchem J, Sherry C, Fines D, Cook MC. Acidosis, lactate, electrolytes, muscle enzymes, and other factors in the blood of Sus scrofa following repeated TASER exposures. Forensic Sci Int. 2006;161:20–30.
Jauchem J, Beason C, Cook M. Acute effects of an alternative electronic control device waveform in swine. Forensic Sci Med Pathol. 2009;5:2–10.
Jauchem J, Cook M, Beason C. Blood factors of Sus scrofa following a series of three TASER electronic control device exposures. Forensic Sci Int. 2008;175:166–70.
Dennis A, Valentino D, Walter R, Nagy K, Winners J, Bokhari F, Wiley D, Joseph K, Roberts R. Acute effects of TASER X26 discharges in a swine model. J Trauma. 2007;63:581–90.
Sanford J, Jacobs G, Roe E, Terndrup T. Two patients subdued with a TASER device: cases and review of complications. J Emerg Med. 2008. doi:10.1016/j.jemermed.2007.10.059.
Bozeman W, Hauda W, Heck J, Graham D, Martin B, Winslow J. Safety and injury profile of conducted electrical weapons used by law enforcement officers against criminal suspects. Ann Emerg Med. 2009;53(4):480–9.
Bui E, Sourkes M, Wennberg R. Generalized tonic-clonic seizure after a TASER shot to the head. CMAJ. 2009;180(6):625–6.
Hick J, Smith S, Lynch M. Metabolic acidosis in restraint-associated cardiac arrest: a case series. Acad Emerg Med. 1999;6(3):239–43.
Merigian K, Roberts J. Cocaine intoxication: hyperpyrexia, rhabdomyolysis, and acute renal failure. Clin Toxicol. 1987;25:135–48.
Menashe P, Gottlieb J. Hyperthermia, rhabdomyolysis, and myoglobinuric renal failure after recreational use of cocaine. South Med J. 1988;81(3):379–81.
Sinert R, Kohl L, Reinone T, Scalea T. Exercise-induced rhabdomyolysis. Ann Emerg Med. 1994;23(6):1301–6.
Lin AC-M, Lin C-M, Wang T-L, Leu J-G. Rhabdomyolysis in 119 students after repetitive exercise. Br J Sports Med. 2005;39:e3.
Welch R, Todd K, Krause G. Incidence of cocaine-associated rhabdomyolysis. Ann Emerg Med. 1991;20(2):154–7.
Goldstein R, DesLauriers C, Burda A. Cocaine: history, social implications, and toxicity—a review. Dis Mon. 2009;55:6–38.
Horowitz B, Panacek E, Jouriles N. Severe rhabdomyolysis with renal failure after intranasal cocaine use. J Emerg Med. 1997;15(6):833–7.
Roth D, Alarcon F, Fernandez J, Preston R, Bourgoignie J. Acute rhabdomyolysis associated with cocaine intoxication. N Engl J Med. 1988;319:673–7.
Albertson T, Derlet R, Van Hoozen B. Methamphetamine and the expanding complications of amphetamines. West J Med. 1999;170:214–9.
Kedrick WC, Hull AR, Knochel JP. Rhabdomyolyis and shock after intravenuous amphetamine administration. Ann Int Med. 1977;86(4):381–7.
Vanholder R, Sever M, Erek E, Lameire N. Rhabdomyolysis. J Am Soc Nephrol. 2000;11:1553–61.
Tan W, Herzlich B, Funaro R, Koutelos K, Pagala M, Amaladevi B, Grob D. Rhabdomyolysis and myoglobinuric acute renal failure associated with classic heat stroke. South Med J. 1995;88(10):1065–8.
Heatstroke and rhabdomyolysis presenting as fulminant hepatic failure. Postgrad Med J. 1988;64(748):157–9.
Enoka RM. Morphologic features and activation patterns of motor units. J Clin Neurophysiol. 1995;12:538–59.
Ding J, Wexler A, Binder-MacLeod SA. A mathematical model that predicts the force-frequency relationship of human skeletal muscle. Muscle Nerve. 2002;26:477–85.
Johnson MA, Polfar J, Weightman D, Appleton D. Data on the distribution of fiber types in thirty-six human muscles: an autopsy study. J Neurol Sci. 1973;18:111–29.
Gregory CM, Bickel CS. Recruitment patterns in human skeletal muscle during electrical stimulation. Phys Ther. 2005;85:358–64.
Conflict of interest
Drs. Dawes is an external medical consultant to TASER International, Inc. Dr. Ho is the medical director for TASER International, Inc. Both Drs. Dawes and Ho are company stockholders. Dr. Sweeney is a member of the scientific and medical advisory board for TASER International. The remaining authors have no conflicts to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dawes, D.M., Ho, J.D., Sweeney, J.D. et al. The effect of an electronic control device on muscle injury as determined by creatine kinase enzyme. Forensic Sci Med Pathol 7, 3–8 (2011). https://doi.org/10.1007/s12024-010-9187-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12024-010-9187-4