Abstract
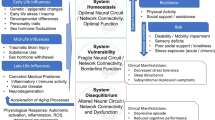
Late life depression is a complex disease associated with a number of contributing neurobiological factors, including cerebrovascular disease, neurodegeneration, and inflammation, which also contribute to its longitudinal prognosis and course. These factors create a context in which the brain is more vulnerable to the impact of stress, and thus, to depression. At the same time, some individuals are protected from late life depression and its consequences, even in the face of neurobiological vulnerability, through benefitting from one or more attributes associated with resilience, including social support, engagement in physical and cognitive activities, and brain reserve. Enhanced understanding of how neurobiological and environmental factors interact in predicting vulnerability and resilience is needed to predict onset and course of depression in late life and develop more effective interventions.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bruce ML, Seeman TE, Merrill SS, Blazer DG. The impact of depressive symptomatology on physical disability: MacArthur Studies of Successful Aging. Am J Public Health. 1994;84:1796–9.
Bruce ML. Depression and disability in late life: directions for future research. Am J Geriatr Psychiatry. 2001;9:102–12.
Gallo JJ, Bogner HR, Morales KH, et al. The effect of a primary care practice-based depression intervention on mortality in older adults: a randomized trial. Ann Intern Med. 2007;146:689–98.
Sheeran T, Brown EL, Nassisi P, Bruce ML. Does depression predict falls among home health patients? Using a clinical-research partnership to improve the quality of geriatric care. Home Healthc Nurse. 2004;22:384–9.
Voaklander DC, Rowe BH, Dryden DM, et al. Medical illness, medication use and suicide in seniors: a population-based case-control study. J Epidemiol Community Health. 2008;62:138–46.
Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961–70.
Aizenstein HJ, Butters MA, Figurski JL, et al. Prefrontal and striatal activation during sequence learning in geriatric depression. Biol Psychiatry. 2005;58:290–6.
Aizenstein HJ, Butters MA, Wu M, et al. Altered functioning of the executive control circuit in late-life depression: episodic and persistent phenomena. Am J Geriatr Psychiatry. 2009;17:30–42.
Post F. The significance of affective disorders in old age. London: Institute of Psychiatry; 1962. Maudsley Monograph.
Post F, Schulman K. New views on old age affective disorder. In: Aire T, editor. Recent advances in psychogeriatrics. New York: Churchill Livingstone Inc; 1985. p. 119–40.
Alexopoulos GS. “The depression-executive dysfunction syndrome of late life”: a specific target for D3 agonists? Am J Geriatr Psychiatry. 2001;9:22–9.
Alexopoulos GS. Frontostriatal and limbic dysfunction in late-life depression. Am J Geriatr Psychiatry. 2002;10:687–95.
Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497–501.
Krishnan KR, Taylor WD, McQuoid DR, et al. Clinical characteristics of magnetic resonance imaging-defined subcortical ischemic depression. Biol Psychiatry. 2004;55:390–7.
Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54:915–22.
Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18:963–74. This seminal review of the vascular depression literature proposes three mechanisms mediating the relationship between vascular disease and depression in late life, including disconnection, hypoperfusion, and inflammation.
Lamar M, Charlton RA, Ajilore O, et al. Prefrontal vulnerabilities and whole brain connectivity in aging and depression. Neuropsychologia. 2013;51:1463–70. Healthy older adults exhibited a significant relationship between white matter network vulnerability as measured using diffusion tensor imaging fractional anisotropy (FA) and performance on the Object Alteration Task. This relationship was not present in older depressed individuals, in which a significant relationship was observed only in FA of the uncinate fasciculus and task performance. The authors conclude that whole brain impact of vulnerabilities in fronto-limbic networks associated with aging may be obscured in the context of disease, such as depression.
Smith EE, Salat DH, Jeng J, et al. Correlations between MRI white matter lesion location and executive function and episodic memory. Neurology. 2011;76:1492–9.
Dalby RB, Frandsen J, Chakravarty MM, et al. Depression severity is correlated to the integrity of white matter fiber tracts in late-onset major depression. Psychiatry Res. 2010;184:38–48.
de Groot E, Hovingh GK, Wiegman A, et al. Measurement of arterial wall thickness as a surrogate marker for atherosclerosis. Circulation. 2004;109(23 Suppl 1):III33–8.
Wong M, Edelstein J, Wollman J, Bond MG. Ultrasonic-pathological comparison of the human arterial wall. Verification of intima-media thickness. Arterioscler Thromb. 1993;13:482–6.
Chen CS, Chen CC, Kuo YT, et al. Carotid intima-media thickness in late-onset major depressive disorder. Int J Geriatr Psychiatry. 2006;21:36–42.
Smith PJ, Blumenthal JA, Babyak MA, et al. Intima-media thickness and age of first depressive episode. Biol Psychol. 2009;80:361–4.
Paranthaman R, Greenstein A, Burns AS, et al. Relationship of endothelial function and atherosclerosis to treatment response in late-life depression. Int J Geriatr Psychiatry. 2012;27:967–73.
Deanfield J, Donald A, Ferri C, et al. Endothelial function and dysfunction. Part I: methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:7–17.
Di Napoli M, Papa F. C-reactive protein and cerebral small-vessel disease: an opportunity to reassess small-vessel disease physiopathology? Circulation. 2005;112:781–5.
Greenstein AS, Paranthaman R, Burns A, et al. Cerebrovascular damage in late-life depression is associated with structural and functional abnormalities of subcutaneous small arteries. Hypertension. 2010;56:734–40.
Paranthaman R, Greenstein AS, Burns AS, et al. Vascular function in older adults with depressive disorder. Biol Psychiatry. 2010;68:133–9.
Direk N, Koudstaal PJ, Hofman A, et al. Cerebral hemodynamics and incident depression: the Rotterdam Study. Biol Psychiatry. 2012;72:318–23. In a large cohort of older adults from the Rotterdam Study, lower blood flow velocity (a proxy for cerebral metabolism), was associated with depressive symptoms and disorders. Reduced vasomotor activity (suggestive of cerebral microangiopathy), was associated with depressive disorders.
Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7:323–31.
Diniz BS, Butters MA, Albert SM, et al. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202:329–35. A meta-analysis of 23 studies to calculate the pooled risk of dementia in late life depression resulted in odds ratios of 1.85 for all cause dementia,1.65 for Alzheimer’s disease, and 2.52 for vascular dementia, respectively.
Geerlings MI, den Heijer T, Koudstaal PJ, et al. History of depression, depressive symptoms, and medial temporal lobe atrophy and the risk of Alzheimer disease. Neurology. 2008;70:1258–64.
Kumar A, Bilker W, Jin Z, Udupa J. Atrophy and high intensity lesions: complementary neurobiological mechanisms in late-life major depression. Neuropsychopharmacology. 2000;22:264–74.
Sexton CE, Mackay CE, Ebmeier KP. A systematic review and meta-analysis of magnetic resonance imaging studies in late-life depression. Am J Geriatr Psychiatry. 2013;21:184–95. A meta-analysis of 17 studies investigating gray matter volumes in late life depression found significantly lower volumes in hippocampus, orbitofrontal cortex, putamen, and thalamus among depressed relative to non-depressed comparison participants.
Steffens DC, Byrum CE, McQuoid DR, et al. Hippocampal volume in geriatric depression. Biol Psychiatry. 2000;48:301–9.
Zahodne LB, Gongvatana A, Cohen RA, Ott BR, Tremont G. Are apathy and depression independently associated with longitudinal trajectories of cortical atrophy in mild cognitive impairment? Am J Geriatr Psychiatry. 2013;21:1098–106. Using a sample of 334 participants with Mild Cognitive Impairment (MCI) from the Alzheimer’s Disease Neuroimaging Initiative Study, investigators found that depression was associated with less cortical thickness in the entorhinal cortex at baseline and greater atrophy over the study period (M = 30.5 months). Apathy was not predictive of cortical thickness in any region measured at baseline or over the study period. Findings suggest that in patients with MCI, depression may be a stronger predictor of longitudinal cortical atrophy than apathy.
Sweet RA, Hamilton RL, Butters MA, et al. Neuropathologic correlates of late-onset major depression. Neuropsychopharmacology. 2004;29:2242–50.
Beekman AT. Neuropathological correlates of late-life depression. Expert Rev Neurother. 2011;11:947–9.
Thomas AJ, Ferrier IN, Kalaria RN, et al. A neuropathological study of vascular factors in late-life depression. J Neurol Neurosurg Psychiatry. 2001;70:83–7.
Rapp MA, Schnaider-Beeri M, Grossman HT, et al. Increased hippocampal plaques and tangles in patients with Alzheimer disease with a lifetime history of major depression. Arch Gen Psychiatry. 2006;63:161–7.
Butters MA, Klunk WE, Mathis CA, et al. Imaging Alzheimer pathology in late-life depression with PET and Pittsburgh Compound-B. Alzheimer Dis Assoc Disord. 2008;22:261–8.
Kumar A, Kepe V, Barrio JR, et al. Protein binding in patients with late-life depression. Arch Gen Psychiatry. 2011;68:1143–50.
Wu C, Pike VW, Wang Y. Amyloid imaging: from benchtop to bedside. Curr Top Dev Biol. 2005;70:171–213.
Madsen K, Hasselbalch BJ, Frederiksen KS, et al. Lack of association between prior depressive episodes and cerebral [11C]PiB binding. Neurobiol Aging. 2012;33:2334–42. Older adults with remitted depression were equivalent to never-depressed comparisons in [11C]PiB binding, but did demonstrate more severe white matter lesions. There were no associations between PiB binding and number of depressive episodes, cognitive performance, or antidepressant treatment. The authors conclude that their findings suggest that history of depression may not be associated with development of Alzheimer’s disease.
Pomara N, Willoughby LM, Sidtis JJ, Mehta PD. Selective reductions in plasma Abeta 1-42 in healthy elderly subjects during longitudinal follow-up: a preliminary report. Am J Geriatr Psychiatry. 2005;13:914–7.
Schupf N, Tang MX, Fukuyama H, et al. Peripheral Abeta subspecies as risk biomarkers of Alzheimer’s disease. Proc Natl Acad Sci. 2008;105:14052–7.
Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59:512–9.
Pomara N, Doraiswamy PM, Willoughby LM, et al. Elevation in plasma Abeta42 in geriatric depression: a pilot study. Neurochem Res. 2006;31:341–9.
Osorio RS, Gumb T, Pomara N. Soluble amyloid-beta levels and late-life depression. Curr Pharm Des. 2014;20:2547–54.
Blasko I, Kemmler G, Jungwirth S, et al. Plasma amyloid beta-42 independently predicts both late-onset depression and Alzheimer disease. Am J Geriatr Psychiatry. 2010;18:973–82.
Sun X, Steffens DC, Au R, et al. Amyloid-associated depression: a prodromal depression of Alzheimer disease? Arch Gen Psychiatry. 2008;65:542–50.
Pomara N, Bruno D, Sarreal AS, et al. Lower CSF amyloid beta peptides and higher F2-isoprostanes in cognitively intact elderly individuals with major depressive disorder. Am J Psychiatry. 2012;169:523–30. In a sample of 47 non-demented older adults, CSF Aβ42 levels were significantly lower and isoprostane levels were significantly higher among those with Major Depressive Disorder relative to the healthy comparison group. There were no group differences in measures of CSF tau. The authors conclude that the reduction in CSF amyloid beta levels in older adults with depression may be due to increased brain beta amyloid plaques or decreased soluble amyloid beta production.
Gudmundsson P, Skoog I, Waern M, et al. The relationship between cerebrospinal fluid biomarkers and depression in elderly women. Am J Geriatr Psychiatry. 2007;15:832–8.
Buerger K, Zinkowski R, Teipel SJ, et al. Differentiation of geriatric major depression from Alzheimer’s disease with CSF tau protein phosphorylated at threonine 231. Am J Psychiatry. 2003;160:376–9.
Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci. 1991;12:383–8.
Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med. 2011;73:114–26.
Wolf OT, Convit A, de Leon MJ, et al. Basal hypothalamo-pituitary-adrenal axis activity and corticotropin feedback in young and older men: relationships to magnetic resonance imaging-derived hippocampus and cingulate gyrus volumes. Neuroendocrinology. 2002;75:241–9.
Green KN, Billings LM, Roozendaal B, et al. Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer’s disease. J Neurosci. 2006;26:9047–56.
Dong H, Yuede CM, Yoo HS, et al. Corticosterone and related receptor expression are associated with increased beta-amyloid plaques in isolated Tg2576 mice. Neuroscience. 2008;155:154–63.
Kang JE, Cirrito JR, Dong H, et al. Acute stress increases interstitial fluid amyloid-beta via corticotropin-releasing factor and neuronal activity. Proc Natl Acad Sci U S A. 2007;104:10673–8.
Alexopoulos GS, Morimoto SS. The inflammation hypothesis in geriatric depression. Int J Geriatr Psychiatry. 2011;26:1109–18.
Baune BT, Smith E, Reppermund S, et al. Inflammatory biomarkers predict depressive, but not anxiety symptoms during aging: the prospective Sydney Memory and Aging Study. Psychoneuroendocrinology. 2012;37:1521–30. Among 1037- non-demented community volunteers, IL-6 and IL-8 levels were associated with depressive symptoms at baseline. IL-8 levels were additionally associated with depressive symptoms at two-year follow-up and the onset of mild to moderate depression over two years. PAI-I was related to remitted depression, and there was no relationship with cytokine levels and anxiety symptoms.
Bremmer MA, Beekman AT, Deeg DJ, et al. Inflammatory markers in late-life depression: results from a population-based study. J Affect Disord. 2008;106:249–55.
Dentino AN, Pieper CF, Rao MK, et al. Association of interleukin-6 and other biologic variables with depression in older people living in the community. J Am Geriatr Soc. 1999;47:6–11.
Dimopoulos N, Piperi C, Psarra V, et al. Increased plasma levels of 8-iso-PGF2alpha and IL-6 in an elderly population with depression. Psychiatry Res. 2008;161:59–66.
Forti P, Rietti E, Pisacane N, et al. Blood inflammatory proteins and risk of incident depression in the elderly. Dement Geriatr Cogn Disord. 2010;29:11–20.
Matheny ME, Miller RR, Shardell MD, et al. Inflammatory cytokine levels and depressive symptoms in older women in the year after hip fracture: findings from the Baltimore Hip Studies. J Am Geriatr Soc. 2011;59:2249–55.
Milaneschi Y, Corsi AM, Penninx BW, et al. Interleukin-1 receptor antagonist and incident depressive symptoms over 6 years in older persons: the InCHIANTI study. Biol Psychiatry. 2009;65:973–8.
Penninx BW, Kritchevsky SB, Yaffe K, et al. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol Psychiatry. 2003;54:566–72.
Naude PJ, Eisel UL, Comijs HC, et al. Neutrophil gelatinase-associated lipocalin: a novel inflammatory marker associated with late-life depression. J Psychosom Res. 2013;75:444–50.
Tiemeier H, Hofman A, van Tuijl HR, et al. Inflammatory proteins and depression in the elderly. Epidemiology. 2003;14:103–7.
Fornage M, Chiang YA, O’Meara ES, et al. Biomarkers of inflammation and MRI-defined small vessel disease of the brain: The Cardiovascular Health Study. Stroke. 2008;39:1952–9.
Raz N, Yang Y, Dahle CL, Land S. Volume of white matter hyperintensities in healthy adults: contribution of age, vascular risk factors, and inflammation-related genetic variants. Biochim Biophys Acta. 1822;2012:361–9. Among 144 healthy older adults, larger white matter hyperintensity volume was associated with age, hypertension, and elevated levels of homocysteine and C-reactive protein, but not with low-density lipoprotein levels. Individuals with genetic variants protomoting inflammation had greater volume of white matter hyperintensities than those who did not.
Satizabal CL, Zhu YC, Mazoyer B, et al. Circulating IL-6 and CRP are associated with MRI findings in the elderly: the 3C-Dijon Study. Neurology. 2012;78:720–7.
van Dijk EJ, Prins ND, Vermeer SE, et al. C-reactive protein and cerebral small-vessel disease: the Rotterdam Scan Study. Circulation. 2005;112:900–5.
Elderkin-Thompson V, Irwin MR, Hellemann G, Kumar A. Interleukin-6 and memory functions of encoding and recall in healthy and depressed elderly adults. Am J Geriatr Psychiatry. 2012;20:753–63. Performance on a memory measure was associated with IL-6 levels among depressed and non-depressed older adults. Older adults with depression had poorer recall than comparisons, independent of IL-6 levels.
Maes M, Leonard BE, Myint AM, et al. The new ‘5-HT’ hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:702–21.
O’Connor JC, Andre C, Wang Y, et al. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J Neurosci. 2009;29:4200–9.
O’Connor JC, Lawson MA, Andre C, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14:511–22.
Stone TW, Behan WM. Interleukin-1beta but not tumor necrosis factor-alpha potentiates neuronal damage by quinolinic acid: protection by an adenosine A2A receptor antagonist. J Neurosci Res. 2007;85:1077–85.
Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105:751–6.
Leonard BE. Inflammation, depression and dementia: are they connected? Neurochem Res. 2007;32:1749–56.
Hermida AP, McDonald WM, Steenland K, Levey A. The association between late-life depression, mild cognitive impairment and dementia: is inflammation the missing link? Expert Rev Neurother. 2012;12:1339–50.
Wirth M, Villeneuve S, Haase CM, et al. Associations between Alzheimer disease biomarkers, neurodegeneration, and cognition in cognitively normal older people. JAMA Neurol. 2013;70:1512–9. 40 % of cognitively normal older adults with no evidence of elevated PiB binding displayed at least one abnormal neurodegernative biomarker (i.e., hippocampal volume, glucose metabolism, and grath matter thickness). Individuals with abnormal cortical thickness had neurodegenerative abnormalities that were similar to patients with Alzheimer’s disease. Greater neurodegenerative abnormalities were associated with poorer memory and executive functioning performance, but not greater PiB binding.
Adams KB, Sanders S, Auth EA. Loneliness and depression in independent living retirement communities: risk and resilience factors. Aging Ment Health. 2004;8:475–85.
Prince MJ, Harwood RH. Thomas A, Mann AH: A prospective population-based cohort study of the effects of disablement and social milieu on the onset and maintenance of late-life depression. The Gospel Oak Project VII. Psychol Med. 1998;28:337–50.
Yang Y. How does functional disability affect depressive symptoms in late life? The role of perceived social support and psychological resources. J Health Soc Behav. 2006;47:355–72.
Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10:819–28.
Wirth M, Haase CM, Villeneuve S, et al. Neuroprotective pathways: lifestyle activity, brain pathology, and cognition in cognitively normal older adults. Neurobiol Aging. 2014;35:1873–82. Among 92 cognitively normal older adults, higher lifetime cognitive activity and higher current physical activity were associated with fewer white matter lesions, which were, in turn, associated with a higher composite measure of global integrity and higher cognitive functioning. Higher lifetime cognitive activity predicted lower PiB retention, which moderated the relationship between neural integiry and cognition. It is concluded that lifetime cognitive and current physical activity may decrease the development of dementia by protecting against cerebrovascular and A beta pathology.
Blake H, Mo P, Malik S, Thomas S. How effective are physical activity interventions for alleviating depressive symptoms in older people? A systematic review. Clin Rehabil. 2009;23:873–87.
Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–60.
Amieva H, Mokri H, Le Goff M, et al. Compensatory mechanisms in higher-educated subjects with Alzheimer’s disease: a study of 20 years of cognitive decline. Brain. 2014;137(Pt 4):1167–75. Over a 20-year period, cognition among 442 people with incident Alzheimer’s disease were assessed. Cognitive symptoms emerge up to 16 years preceding conversion to dementia in high and low educated individuals, but among those with low education, more immediately converted to dementia, whereas conversion was slower among those with higher levels of education.
Osone A, Arai R, Hakamada R, Shimoda K. Impact of cognitive reserve on the progression of mild cognitive impairment to Alzheimer’s disease in Japan. Geriatr Gerontol Int. 2014. doi:10.1111/ggi.12292.
Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–28.
Paulson D, Bowen ME, Lichtenberg PA. Does brain reserve protect older women from vascular depression? J Gerontol B Psychol Sci Soc Sci. 2014;69:157–67. Among 1355 older women, higher level of education and lower cerebrovascular burden were negatively associated with depressive symptoms at baseline, but over six years, the protective effect of education on depressive symptoms decreased.
Compliance with Ethics Guidelines
Conflict of Interest
Anand Kumar declares no conflict of interest.
Sara L. Weisenbach has received a grant from the Department of Veterans Affairs (Career Development Award)
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Support for this work comes from a Level-2 Career Development Award from the Department of Veterans Affairs, Rehabilitation Research & Development to SLW.
This article is part of the Topical Collection on Geriatric Disorders
Rights and permissions
About this article
Cite this article
Weisenbach, S.L., Kumar, A. Current Understanding of the Neurobiology and Longitudinal Course of Geriatric Depression. Curr Psychiatry Rep 16, 463 (2014). https://doi.org/10.1007/s11920-014-0463-y
Published:
DOI: https://doi.org/10.1007/s11920-014-0463-y