Abstract
Sepsis is a common and high-burden healthcare problem with a mortality exceeding 20 % in severe sepsis and nearly 50 % when septic shock is present. Early goal-directed therapy (EGDT) is recommended by sepsis guidelines as the standard of care following a landmark study by Rivers et al. alongside other observational studies. Three recent randomized controlled trials have questioned the Rivers’ results. The objective of our systematic review was to assess the effectiveness of EGDT in reducing the mortality of severe sepsis or septic shock. Relevant primary studies were identified by searching the MEDLINE and EMBASE databases and the Cochrane Central Register of Controlled Clinical Trials to identify randomized controlled trials assessing the effectiveness of EGDT for sepsis. Data from all trials were combined and analyzed using a random effects model. Five studies, enrolling a total of 4033 patients, were included in the meta-analysis. In-hospital mortality did not differ between the two treatment groups (RR 0.93, 95 % CI 0.77–1.11, P = 0.42), although moderate heterogeneity between studies was noted (I 2 = 48 %). A non-significant trend toward reduction in 60-day mortality in the EGDT group was noted (RR 0.93, 95 % CI 0.82–1.05, P = 0.22, I 2 = 24 %). Heterogeneity between trials precludes a definitive conclusion on the utility of EGDT in severe sepsis. Until further evidence is available, it is reasonable to consider EGDT in the care of patients with severe sepsis and septic shock.
Similar content being viewed by others
Introduction
Sepsis is a systemic, deleterious host response to infection [1]. Severe sepsis and septic shock are major healthcare problems, with reported annual incidence nearing 300 cases per 100,000 adults [2–4]. Despite decreasing mortality in recent years [5], short-term mortality remains 20 % or more [6], reaching up to 50 % when shock is present [7, 8].
Most patients who present with sepsis receive initial care in the emergency department. Similar to polytrauma, acute myocardial infarction, or stroke, the speed and appropriateness of therapy administered in the initial hours after severe sepsis develops are likely to influence outcome [1].
In 2001, a single-center randomized controlled trial by Rivers et al. demonstrated a reduction in mortality (30.5 vs 46.5 %) among patients with severe sepsis and septic shock who were treated according to a 6-hour protocol of early goal-directed therapy (EGDT) when compared to standard therapy [9]. Starting from the hypothesis that usual care lacked aggressive, timely assessment and treatment, the EGDT protocol provided for central venous catheterization to monitor central venous pressure (CVP) and central venous oxygen saturation (Scvo2), which were used to guide the infusion of intravenous fluids, vasopressors, packed red cell transfusions, and inotropic agents, to achieve pre-specified physiological targets.
Due to this study, and a number of nonrandomized studies showing a survival benefit with bundle-based care that included EGDT [10–13], EGDT was ultimately incorporated into the 6-hour resuscitation bundle of the Surviving Sepsis Campaign guidelines as “strong recommendation, low quality of evidence” [1, 14, 15].
Despite such successes, considerable controversy has surrounded the role of EGDT in the treatment of patients with severe sepsis. Concerns have included threats to external validity of the original trial as well as the infrastructure and resource required for implementing EGDT [16, 17]. Other authors also underline issues concerning the bundling of therapies in the EGDT protocol; some components may cause harm (e.g., central line insertion, blood transfusion), other components may fail to incorporate up-to-date views of cardiovascular physiology (e.g., targeting a CVP of 8–12 mm Hg) [18].
Furthermore, in the time since the Rivers publication, the standard of care of sepsis has changed, with emphasis on early identification and treatment spawned by the Surviving Sepsis Campaign. This has led some authors to question whether all the elements of the original protocol are still necessary [19–21].
Recently, three large multicenter randomized controlled trials, one conducted in 31 academic centers in the United States [22], the second in 51 centers, mostly in Australia or New Zealand [23], and the last in 56 hospitals in England [24], have compared the EGDT protocol to usual care and failed to identify a survival benefit.
Due to conflicting data on EGDT effectiveness and the statistical power limitations of individual studies, we decided to perform a systematic review of randomized controlled trials comparing the efficacy of EGDT to usual care in the treatment of septic shock.
Methods
Types of studies
Randomized controlled trials (RCTs) assessing the effectiveness of EGDT vs usual care in the treatment of septic shock.
Types of participants
18 years of age or older, sepsis suspected by treating physician, and presence of septic shock, as defined by each study.
Types of interventions
Trials comparing the original Rivers EGDT protocol with usual care.
In particular, the original Rivers EGDT protocol [9] provided for: early placement of a central venous catheter capable of measuring central venous oxygen saturation and central venous pressure; crystalloid fluid infusion (500 mL bolus given every 30 min to achieve a central venous pressure of 8–12 mmHg); vasopressor infusion to maintain a mean arterial pressure of ≥65 mmHg; packed red cell transfusion if central venous oxygen saturation <70 % to achieve a hematocrit of ≥30 %; dobutamine administration if central venous oxygen saturation <70 %; continuous monitoring of patients’ temperature, heart rate, urine output, blood pressure, and central venous pressure for the first 6 h; monitoring of arterial and venous blood gas values, lactate concentrations, and coagulation-related variables (see Fig. 6 in “Appendix”).
Outcomes measured
Our primary outcome was all-cause in-hospital mortality, calculated at the longest follow-up available for each study.
Our secondary outcome was all-cause mortality at the longest study follow-up.
Search methods for identification of studies
Relevant primary studies were identified by searching the MEDLINE and EMBASE databases, the Cochrane Central Register of Controlled Clinical Trials (CENTRAL), and http://Clinicaltrials.gov database to identify ongoing trials yet to be reported in the literature up to April 1, 2015. Reference lists included in clinical guidelines and the proceedings of relevant meetings were also considered.
We performed the research following the P.I.C.O.s. acronym:
-
1.
Population: adult patients (over 18 years of age) with sepsis, severe sepsis, or septic shock.
-
2.
Intervention: early goal-directed therapy, defined on the basis of the “Rivers protocol” (see Fig. 6 in “Appendix”).
-
3.
Comparison: usual care or standard therapy (not included as a keyword in the search so as to increase search sensitivity).
-
4.
Outcome: mortality (not included as a keyword in the search so as to increase search sensitivity).
-
5.
Type of study: randomized controlled trials.
The search strategy was:
(((((((((sepsis) OR septicemia) OR septic shock) OR severe sepsis) OR blood stream infection) OR endotoxic shock) OR toxic shock)) AND ((((((((egdt) OR early goal-directed therapy) OR early goal therapy) OR early directed therapy) OR protocol directed therapy) OR goal-directed therapy) OR rivers protocol) OR protocol)) AND ((((((randomized controlled trial) OR controlled clinical trial) OR randomized) OR randomly) OR trial) OR groups).
No language restrictions were applied.
Two reviewers (AMR and IB) independently screened titles and abstracts to identify relevant publications. Full texts were retrieved and evaluated by the same two reviewers (AMR and IB) and a final decision regarding the inclusion or exclusion of the papers was made. Discrepancies were resolved by consensus between the two reviewers (AMR and IB).
Data extraction
Two reviewers (AMR and IB) extracted the data, which were then recorded in an electronic spreadsheet. Extracted data consisted of the study characteristics (first author, journal and year of publication, number of patients enrolled, primary end point, study duration), patient characteristics (mean age, gender, vital signs at enrollment, APACHE II score, therapy given within the first 6 h) and the outcome of interest. Mortality was determined by number of fatalities reported in the studies. If unavailable, data were extracted from the Kaplan–Meier curve.
Assessment of risk of bias in included studies
We assessed risk of bias using the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 [25].
Measures of treatment effect
We used risk ratio (RR) with 95 % confidence intervals (CI) for reporting dichotomous data.
Assessment of heterogeneity
Statistical heterogeneity across studies was tested using the I 2 statistic. Heterogeneity was suggested if P ≤ 0.10. For the interpretation of I 2 we used the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 [26]:
-
0 to 40 %: might not be important.
-
30 to 60 %: may represent moderate heterogeneity.
-
50 to 90 %: may represent substantial heterogeneity.
-
75 to 100 %: considerable heterogeneity.
Data synthesis
Data from all trials were combined using Review Manager 5.3 software program [27]. We used DerSimonian and Laird random effects method to pool data [28].
Results
Results of the search
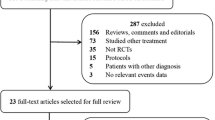
The electronic search yielded 3551 citations: 1115 references were found in MEDLINE and 2436 in EMBASE. 649 references were duplicated between the two databases. No additional references were obtained from the Cochrane trial register or from bibliographic searching of relevant articles. Of a total of 2902 references, 2885 were excluded by title and abstract screening. Of the 17 studies identified as relevant for the meta-analysis, three were excluded because they were reviews [29–31], one was excluded because it was not a randomized controlled trial [32], two were excluded for using a simplified treatment protocol other than the “Rivers protocol” [33, 34], and one was excluded because it was a duplicate report of an included study [35]. Three additional references were excluded because they were statistical analysis plans of enrolled trials [36–38]. Of the remaining seven studies, three were in Chinese [39–41]. We wrote to the authors for more information and data about their studies, but only one replied [39]. Therefore, two studies were excluded due to language restrictions. Ultimately, five studies [9, 22–24, 39] were included in the meta-analysis (Fig. 1). The characteristics of the populations and interventions in the included trials are reported under characteristics of included studies and in Tables 1, 2, and 3.
There was 100 % agreement between the two reviewers on exclusion and inclusion of studies.
Characteristics of included studies
Population
The Rivers et al. trial [9] was performed from March 1997 through March 2000 in a single academic tertiary-level care emergency department in Detroit (United States of America). The second included study, by Wang et al. [39], was performed in a single center in China (Department of Critical Care Medicine of Binzhou, Medical University Hospital, Shandong). The third included study, by Yealy et al. [22], was a multicenter randomized controlled trial conducted from March 2008 through May 2013 in 31 academic emergency departments in the United States of America. Peake et al. [23] conducted a multicenter randomized controlled trial in 51 tertiary and non-tertiary-level metropolitan and rural hospitals, mostly in Australia or New Zealand (6 centers were in Finland, Hong Kong and the Republic of Ireland), from October 2008 through April 2014. Finally, the study by Mouncey et al. [24] was a multicenter randomized controlled trial conducted in 56 hospitals in England, from February 2011 through July 2014.
The four larger trials [9, 22–24] included adult patients in the emergency department who met two or more criteria for systemic inflammatory response syndrome [42], and who had refractory hypotension (systolic blood pressure <90 mmHg after an intravenous fluid challenge) or hypoperfusion (blood lactate level ≥4.0 mmol/L). In the Rivers trial [9], patients who presented to the ED meeting inclusion criteria were enrolled. In the study by Yealy et al. [22] patients did not have to be in shock on arrival in the ED, but could be enrolled within 2 h of detection of shock and within 12 h after arrival. Peake et al. and Mouncey et al. [23, 24] enrolled patients who met the eligibility criteria within 6 h of ED presentation. The criteria for enrollment of patients in septic shock by Wang et al. [39] were not clearly delineated in the information given by the author (contacted by email), and further clarification was hindered due to non-English text.
The number of patients recruited in each of the trials ranged from 33 [39] to 1591 [23]. The total number of patients in the review was 4033, 2003 in the EGDT group and 2030 in the usual care group. There were similar number of patients recruited in both study groups in all trials. Descriptive characteristics of the populations enrolled in the five studies are shown in Table 1.
Interventions
All included trials compared EGDT with usual care. All studies claim to follow Rivers’ original protocol (see Fig. 6 in “Appendix”). In one study [22], there was also a third treatment arm, randomized to a non-EGDT protocol-based treatment. This arm was not included in our review.
Therapies administered in the first 6 hours from enrollment are shown in Table 2.
Outcomes
In the study by Rivers et al. [9], the primary outcome was in-hospital mortality up to 60 days, and is significantly lower in the EGDT group (30.5 vs 46.5 %). Wang et al. [39] also reported a reduction in their primary outcome of 14-day mortality in the EGDT group (25.0 vs 41.2 %).
In the other three enrolled studies, mortality was not significantly affected by EGDT treatment. In particular, Yealy et al. [22] considered hospital mortality up to 60 days as a primary outcome (21.0 vs 18.9 % in the EGDT vs usual care group, respectively), while Peake et al. and Mouncey et al. [23, 24] considered 90-day mortality as their primary outcome (18.6 vs 18.8 % and 29.5 vs 29.2 % in the EGDT vs usual care group, respectively).
The main secondary outcomes of the studies were duration of organ support or length of hospital stay. For more details about outcomes, see Table 3.
Risk of bias in included studies
All included studies stated that the allocation of treatment to patients was randomized. Due to the nature of the intervention, blinding was not possible. However, the outcome (mortality) was not likely to be influenced by lack of blinding [25].
Of the four included studies for which full text analysis was possible, all were deemed by the authors to be at low risk of bias [9, 22–24]. One trial was considered at unclear risk of bias [39], due to inability to analyze the full text because of a language barrier (trial in Chinese).
The summary of risk of bias assessment can be found in Figs. 2 and 3. For more details about support for authors’ judgment see Table 4 in “Appendix”.
We did not investigate potential publication bias by funnel plot [43], due to the small number of included studies.
Effects of interventions
Primary outcome
In-hospital mortality data were available for all studies. 60-day mortality data were available for four trials [9, 22–24]. Wang et al. [39] only reported 14-day mortality.
Overall, EGDT did not reduce in-hospital mortality (RR 0.93, 95 % CI 0.77–1.11, P = 0.42). The analysis showed moderate heterogeneity between studies (I 2 = 48 %) (Fig. 4).
Secondary outcome
Of the four larger studies [9, 22–24], the longest available time point for which mortality could be derived from the respective survival curves was 60 days. This comprised the secondary outcome of our study.
For three studies [22–24], we obtained the data from the respective Kaplan–Meier curves.
Overall, EGDT shows a non-significant trend toward reducing 60-day mortality (RR 0.93, 95 % CI 0.82–1.05, P = 0.22). Heterogeneity between studies for this outcome is not important (I 2 = 24 %) (Fig. 5).
Discussion
The objective of our meta-analysis was to verify the effectiveness of the ‘Rivers protocol’ in septic shock. Considering in-hospital mortality, no significant difference is noted between the two treatment groups, but a moderate heterogeneity between studies is noted (I 2 = 48 %). When comparing mortality at 60 days, a trend toward reduction in mortality for the EGDT protocol emerges, with low study heterogeneity.
These results do not allow us to draw any definitive conclusions regarding EGDT effectiveness in septic shock, given the low number of studies and the heterogeneity among them.
It is strange that there is heterogeneity between trials given the relative similarity of enrollment criteria and treatment protocols. Meanwhile, however, mortality rates differ greatly among the usual care groups, with a higher mortality in the Rivers and Wang studies compared to the Yealy, Peake and Mouncey studies (in-hospital mortality 46 and 41 %, respectively, vs 18.9, 15.7 and 24.6 %), could be considered an indicator of a clinical heterogeneity of patients included in the studies. Moreover looking at the descriptive characteristics of the patients, it could be argued that patients enrolled in the Rivers trial differed, in that they were older compared to Yealy and Peake patients, had more co-morbidities, and had higher lactate levels compared to Yealy, Peake, and Mouncey patients (Table 1). Even patients enrolled in the Wang study, although much younger (mean age 33–36 years), had characteristics of more severe disease (higher APACHE II score, lactate even higher than Rivers’ study). Given that EGDT performed relatively favorably in these two studies, it might suggest that EGDT provides a more substantial benefit in patients who are sicker upon presentation.
Additionally, therapies administered in the first 6 hours from enrollment vary widely between the five trials, both in the EGDT and the usual care groups (Table 2). We wonder if these differences can be due to a different application of the treatment protocol or to different kinds of enrolled patients. Indeed, in the 14 years that separate these studies, many practices have changed in the treatment of sepsis. The Surviving Sepsis Campaign guidelines [1] encompass many elements of the Rivers protocol, and they have likely influenced the usual care of sepsis and septic shock since that initial study. Accordingly, examination of treatment differences in the usual care groups of the included studies reveals significant changes over time. For example, patients in the recently published studies [22–24], receive significantly greater volumes of fluid than the Rivers and Wang studies. Heightened search for sepsis and increasingly prompt initiation of therapy have certainly occurred since the initial 2001 study [44].
Other elements not provided by the original Rivers protocol, such as the early administration of antibiotics, have become part of common clinical practice, and have been shown to significantly improve mortality in patients with septic shock [12, 45–50].
Furthermore, a notable problem is that the classic EGDT protocol includes complex interventions and monitoring. This complexity makes it difficult to distinguish which elements of the protocol have the greatest effect on patient prognosis. It may be possible that early and rapid fluid challenge targeted to key, readily available clinical and hemodynamic parameters (such as systolic blood pressure, the clearance of lactate or inferior vena cava diameter), is one of most beneficial components of the bundle. Data from these trials suggest that aggressive fluid resuscitation has improved in the subsequent years since the initial study.
It is also possible that a benefit of EGDT is not due to any single component, but rather the collective benefit of a sequence of well-defined clinical targets and therapies that emphasize close attention to hemodynamic variables and the rapid correction of physiologic disorder.
Indeed, a recent meta-analysis of 13 randomized controlled trials shows that goal-directed therapy (GDT) of any type (not only the Rivers protocol as assessed in our review) is associated with a significant reduction in overall mortality in patients with sepsis [51]. This mortality benefit is present only in studies starting GDT early but not when initiated late or with unclear timing of GDT. The included studies spanned 22 years (1992–2014), with high heterogeneity of included populations and protocols. The meta-analysis suffers from high clinical heterogeneity, likely due to constantly evolving concepts of sepsis management over the 22-year inclusion period. We believe that improvements in therapeutic approaches and standard of care over a period of 22 years may dwarf potential benefits of any single study intervention.
Limitations of the review
Our review has obvious limitations. First, there are only five including studies, as there are very few published studies investigating the initial Rivers protocol. Language barriers prevented us from including two Chinese trials [40, 41] assessing our outcomes of interest. Furthermore, mortality data at 60 days for three studies [22–24] were derived from Kaplan–Meier estimates instead of raw data.
Conclusion
The high heterogeneity between the trials does not permit a definitive conclusion of the utility of EGDT in severe sepsis and septic shock. This is partially attributable to the inclusion of aspects of EGDT into the usual care of sepsis over the past decade. This has likely created a significant effect in reducing mortality and reducing the potential treatment difference between patients receiving protocolized EGDT and those treated with usual care.
Until further evidence exists, it is still reasonable to consider EGDT, although strict adherence to the original EGDT protocol does not appear necessary. It appears likely that rapid identification of sepsis, early intervention of hemodynamic support with fluids, prompt administration of appropriate antimicrobial therapy and monitoring of clinical and hemodynamic parameters (blood pressure, urine output, lactate), are the key elements to be considered in the treatment of patients with severe sepsis or septic shock, especially in patients with a high baseline risk of mortality.
References
Dellinger RP, Levy MM, Rhodes A et al (2013) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock 2012. Crit Care Med 41(2):580–637
Jawad I, Lukšić I, Rafnsson SB (2012) Assessing available information on the burden of sepsis: global estimates of incidence, prevalence and mortality. J Glob Health 2(1):010404
Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29(7):1303–1310
Gaieski DF, Edwards JM, Kallan MJ, Carr BG (2013) Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 41:1167–1174
Kaukonen K-M, Bailey M, Suzuki S, Pilcher D, Bellomo R (2014) Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA 311:1308–1316
Angus DC, van der Poll T (2013) Severe sepsis and septic shock. N Engl J Med 369(9):840–851
Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29:1303–1310
Annane D, Bellissant E, Cavaillon JM (2005) Septic shock. Lancet 365:63–78
Rivers E, Nguyen B, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M, Early Goal-Directed Therapy Collaborative Group (2001) Early goal-directed therapy in the treatment of severe sepsis and septic shock. NEJM 345(19):1368–1377
Kortgen A, Niederprüm P, Bauer M (2006) Implementation of an evidence-based “standard operating procedure” and outcome in septic shock. Crit Care Med 34:943–949
Jones AE, Focht A, Horton JM, Kline JA (2007) Prospective external validation of the clinical effectiveness of an emergency department- based early goal-directed therapy protocol for severe sepsis and septic shock. Chest 132:425–432
Barochia AV, Cui X, Vitberg D et al (2010) Bundled care for septic shock: an analysis of clinical trials. Crit Care Med 38:668–678
Levy MM, Dellinger RP, Townsend SR et al (2010) The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 36:222–231
Dellinger RP, Carlet JM, Masur H et al (2004) Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 32:858–873
Dellinger RP, Levy MM, Carlet JM et al (2008) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med 34:17–60
Peake S, Webb S, Delaney A (2007) Early goal-directed therapy of septic shock: we honestly remain skeptical. Crit Care Med 35:994–995
Perel A (2008) Bench-to-bedside review: the initial hemodynamic resuscitation of the septic patient according to Surviving Sepsis Campaign guidelines—does one size fit all? Crit Care 12:223
Schmidt GA (2010) Counterpoint: adherence to early goal-directed therapy: does it really matter? No. Both risks and benefits require further study. Chest 138(3):480–483
Carlbom DJ, Rubenfeld GD (2007) Barriers to implementing protocol-based sepsis resuscitation in the emergency department—results of a national survey. Crit Care Med 35(11):2525–2532
Jones AE, Shapiro NI, Roshon M (2007) Implementing early goal-directed therapy in the emergency setting: the challenges and experiences of translating research innovations into clinical reality in academic and community settings. Acad Emerg Med 14(11):1072–1078
Reade MC, Huang DT, Bell D, Coats TJ, Cross AM, Moran JL, Peake SL, Singer M, Yealy DM, Angus DC (2010) Variability in management of early severe sepsis. Emerg Med J 27(2):110–115
Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, Filbin MR, Shapiro NI, Angus DC (2014) A randomized trial of protocol-based care for early septic shock. ProCESS investigators. N Engl J Med 370(18):1683–1693
Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, Higgins AM, Holdgate A, Howe BD, Webb SA, Williams P (2014) Goal-directed resuscitation for patients with early septic shock. N Engl J Med 371(16):1496–1506
Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, Jahan R, Harvey SE, Bell D, Bion JF, Coats TJ, Singer M, Young JD, Rowan KM, Investigators ProMISe Trial (2015) Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 372(14):1301–1311
Higgins JPT, Altman DG, Stern JAC (eds) (2011) Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from http://www.cochrane-handbook.org. Accessed 1 April 2014
Higgins JPT, Altman DG, Stern JAC (eds) (2011) Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from http://www.cochrane-handbook.org. Accessed 1 April 2014
Review Manager (2014) (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration
DerSimonian R, Laid N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–178
Rinaldi L, Girardis M (2007) A modified goal-directed protocol improves clinical outcome in intensive care unit patients with septic shock patients: a randomized controlled trial. Shock 28(6):741
Qinlan M (2014) A randomized trial of protocol-based care for early septic shock. J Emerg Med 47(2):256–257
Trzeciak S (2014) Protocol-based care for early septic shock. N Engl J Med 371(4):385
El Akabawy HAE, Khalaf M, Ragab F, Naeem M (2011) The concept of early goal-directed therapy in sepsis syndrome. Intensive Care Med 37:S231
Lin SM, Huang CD, Lin HC, Liu CY, Wang CH, Kuo HP (2006) A modified goal-directed protocol improves clinical outcomes in intensive care unit patients with septic shock: a randomized controlled trial. Shock 26(6):551–557
Andrews B, Muchemwa L, Kelly P, Lakhi S, Heimburger DC, Bernard GR (2014) Simplified severe sepsis protocol: a randomized controlled trial of modified early goal-directed therapy in Zambia. Crit Care Med 42(11):2315–2324
Muck A, Adams BD, Howe B (2015) Early goal-directed therapy did not reduce mortality more than usual care in early septic shock. Ann Intern Med 162(6):JC4
Pike F, Yealy DM, Kellum JA, Huang DT, Barnato AE, Eaton TL, Angus DC, Weissfeld LA, Investigators ProCESS (2013) Protocolized care for early septic shock (ProCESS) statistical analysis plan. Crit Care Resusc 15(4):301–310
Delaney A, Peake SL, Bellomo R, Cameron P, Holdgate A, Howe B, Higgins A, Presneill J, Webb S, Investigators ARISE (2013) Australasian resuscitation in sepsis evaluation trial statistical analysis plan. Emerg Med Australas 25(5):406–415
Power GS, Harrison DA, Mouncey PR, Osborn TM, Harvey SE, Rowan KM (2013) The protocolised management in sepsis (ProMISe) trial statistical analysis plan. Crit Care Resusc 15(4):311–317
Wang XZ, Lü CJ, Gao FQ, Li XH, Yan WF, Ning FY (2006) Efficacy of goal-directed therapy in the treatment of septic shock. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 18(11):661–664
Chen ZQ, Jin YH, Chen H, Fu WJ, Yang H, Wang RT (2007) Early goal-directed therapy lowers the incidence, severity and mortality of multiple organ dysfunction syndrome. Nan Fang Yi Ke Da Xue Xue Bao 27(12):1892–1895
Yan J (2010) The effect of early goal-directed therapy on treatment of critical patients with severe sepsis/septic shock: a multi-center, prospective, randomized, controlled study. Chin Crit Care Med 22(6):331–334
Bone RC, Balk RA, Cerra FB et al (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101(6):1644–1655
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple graphical test. BMJ 315:629–634
Lilly CM (2014) The ProCESS trial—a new era of sepsis management. N Engl J Med 370(18):1750–1751
Levy MM, Dellinger RP, Townsend SR et al (2010) Surviving Sepsis Campaign: the surviving sepsis campaign results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med 38:367–374
Kumar A, Roberts D, Wood KE et al (2006) Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34:1589–1596
Castellanos-Ortega A, Suberviola B, García-Astudillo LA et al (2010) Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three-year follow up quasi-experimental study. Crit Care Med 38:1036–1043
Puskarich MA, Trzeciak S, Shapiro NI, Emergency Medicine Shock Research Network (EMSHOCKNET) et al (2011) Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med 39:2066–2071
El Solh AA, Akinnusi ME, Alsawalha LN et al (2008) Outcome of septic shock in older adults after implementation of the sepsis “bundle”. J Am Geriatr Soc 56:272–278
Gurnani PK, Patel GP, Crank CW et al (2010) Impact of the implementation of a sepsis protocol for the management of fluid-refractory septic shock: a single-center, before-and-after study. Clin Ther 32:1285–1293
Gu WJ, Wang F, Bakker J, Tang L, Liu JC (2014) The effect of goal-directed therapy on mortality in patients with sepsis—earlier is better: a meta-analysis of randomized controlled trials. Crit Care 18(5):570
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with human and animals performed by any of the authors.
Informed consent
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rusconi, A.M., Bossi, I., Lampard, J.G. et al. Early goal-directed therapy vs usual care in the treatment of severe sepsis and septic shock: a systematic review and meta-analysis. Intern Emerg Med 10, 731–743 (2015). https://doi.org/10.1007/s11739-015-1248-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-015-1248-y