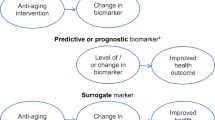
Abstract
Recent advances indicate that biological aging is a potentially modifiable driver of late-life function and chronic disease and have led to the development of geroscience-guided therapeutic trials such as TAME (Targeting Aging with MEtformin). TAME is a proposed randomized clinical trial using metformin to affect molecular aging pathways to slow the incidence of age-related multi-morbidity and functional decline. In trials focusing on clinical end-points (e.g., disease diagnosis or death), biomarkers help show that the intervention is affecting the underlying aging biology before sufficient clinical events have accumulated to test the study hypothesis. Since there is no standard set of biomarkers of aging for clinical trials, an expert panel was convened and comprehensive literature reviews conducted to identify 258 initial candidate biomarkers of aging and age-related disease. Next selection criteria were derived and applied to refine this set emphasizing: (1) measurement reliability and feasibility; (2) relevance to aging; (3) robust and consistent ability to predict all-cause mortality, clinical and functional outcomes; and (4) responsiveness to intervention. Application of these selection criteria to the current literature resulted in a short list of blood-based biomarkers proposed for TAME: IL-6, TNFα-receptor I or II, CRP, GDF15, insulin, IGF1, cystatin C, NT-proBNP, and hemoglobin A1c. The present report provides a conceptual framework for the selection of blood-based biomarkers for use in geroscience-guided clinical trials. This work also revealed the scarcity of well-vetted biomarkers for human studies that reflect underlying biologic aging hallmarks, and the need to leverage proposed trials for future biomarker discovery and validation.


Similar content being viewed by others
References
(IOM) (2010) Evaluation of biomarkers and surrogate endpoints in chronic disease. 2101 CONSTITUTION AVE, WASHINGTON, DC 20418 USA
Abualsuod A, Rutland JJ, Watts TE, Pandat S, Delongchamp R, Mehta JL (2015) The effect of metformin use on left ventricular ejection fraction and mortality post-myocardial infarction. Cardiovasc Drugs Ther 29:265–275. https://doi.org/10.1007/s10557-015-6601-x
Administration FaD (2018) Early Alzheimer’s disease: developing drugs for treatment guidance for industry. Rockville, MD
Allard JS, Perez EJ, Fukui K, Carpenter P, Ingram DK, Cabo R (2015) Prolonged metformin treatment leads to reduced transcription of Nrf2 and neurotrophic factors without cognitive impairment in older C57BL/6J mice. Behav Brain Res 301:1–9. https://doi.org/10.1016/j.bbr.2015.12.012
Andreassen M, Raymond I, Kistorp C, Hildebrandt P, Faber J, Kristensen LO (2009) IGF1 as predictor of all-cause mortality and cardiovascular disease in an elderly population. Eur J Endocrinol 160:25–31. https://doi.org/10.1530/EJE-08-0452
Baker GT III, Sprott RL (1988) Biomarkers of aging. Exp Gerontol 23:223–239
Bannister CA, Holden SE, Jenkins-Jones S, Morgan CL, Halcox JP, Schernthaner G, Mukherjee J, Currie CJ (2014) Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes Metab 16:1165–1173. https://doi.org/10.1111/dom.12354
Barron E, Lara J, White M, Mathers JC (2015) Blood-borne biomarkers of mortality risk: systematic review of cohort studies. PLoS One 10:e0127550. https://doi.org/10.1371/journal.pone.0127550
Bartke A, Brown-Borg H, Mattison J, Kinney B, Hauck S, Wright C (2001) Prolonged longevity of hypopituitary dwarf mice. Exp Gerontol 36:21–28
Barzilai N, Banerjee S, Hawkins M, Chen W, Rossetti L (1998) Caloric restriction reverses hepatic insulin resistance in aging rats by decreasing visceral fat. J Clin Invest 101:1353–1361. https://doi.org/10.1172/JCI485
Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA (2016) Metformin as a tool to target aging. Cell Metab 23:1060–1065. https://doi.org/10.1016/j.cmet.2016.05.011
Batandier C, Guigas B, Detaille D, El-Mir MY, Fontaine E, Rigoulet M, Leverve XM (2006) The ROS production induced by a reverse-electron flux at respiratory-chain complex 1 is hampered by metformin. J Bioenerg Biomembr 38:33–42. https://doi.org/10.1007/s10863-006-9003-8
Bauskin AR et al (2005) The propeptide mediates formation of stromal stores of PROMIC-1: role in determining prostate cancer outcome. Cancer Res 65:2330–2336. https://doi.org/10.1158/0008-5472.CAN-04-3827
Belsky DW et al (2015) Quantification of biological aging in young adults. Proc Natl Acad Sci USA 112:E4104–E4110. https://doi.org/10.1073/pnas.1506264112
Belsky DW, Huffman KM, Pieper CF, Shalev I, Kraus WE (2017a) Change in the rate of biological aging in response to caloric restriction: CALERIE biobank analysis. J Gerontol A Biol Sci Med Sci 73:4–10. https://doi.org/10.1093/gerona/glx096
Belsky DW, Moffitt TE, Cohen AA, Corcoran DL, Levine ME, Prinz JA, Schaefer J, Sugden K, Williams B, Poulton R, Caspi A (2017b) Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: do they measure the same thing? Am J Epidemiol. https://doi.org/10.1093/aje/kwx346
Biomarkers Definitions Working G (2001) Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 69:89–95. https://doi.org/10.1067/mcp.2001.113989
Blackburn EH (2000) Telomere states and cell fates. Nature 408:53–56. https://doi.org/10.1038/35040500
Blasco MA (2007) Telomere length, stem cells and aging. Nat Chem Biol 3:640–649. https://doi.org/10.1038/nchembio.2007.38
Braunwald E (2008) Biomarkers in heart failure. N Engl J Med 358:2148–2159. https://doi.org/10.1056/NEJMra0800239
Breese CR, Ingram RL, Sonntag WE (1991) Influence of age and long-term dietary restriction on plasma insulin-like growth factor-1 (IGF-1), IGF-1 gene expression, and IGF-1 binding proteins. J Gerontol 46:B180–B187
Bridges HR, Jones AJ, Pollak MN, Hirst J (2014) Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem J 462:475–487. https://doi.org/10.1042/BJ20140620
Brown DA, Breit SN, Buring J, Fairlie WD, Bauskin AR, Liu T, Ridker PM (2002) Concentration in plasma of macrophage inhibitory cytokine-1 and risk of cardiovascular events in women: a nested case-control study. Lancet 359:2159–2163. https://doi.org/10.1016/S0140-6736(02)09093-1
Brown DA et al (2006) Measurement of serum levels of macrophage inhibitory cytokine 1 combined with prostate-specific antigen improves prostate cancer diagnosis. Clin Cancer Res 12:89–96. https://doi.org/10.1158/1078-0432.CCR-05-1331
Brown-Borg HM, Bartke A (2012) GH and IGF1: roles in energy metabolism of long-living GH mutant mice. J Gerontol A Biol Sci Med Sci 67:652–660. https://doi.org/10.1093/gerona/gls086
Bruunsgaard H, Andersen-Ranberg K, Hjelmborg J, Pedersen BK, Jeune B (2003) Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am J Med 115:278–283
Burch JB et al (2014) Advances in geroscience: impact on healthspan and chronic disease. J Gerontol A Biol Sci Med Sci 69(Suppl 1):S1–S3. https://doi.org/10.1093/gerona/glu041
Burgers AM et al (2011) Meta-analysis and dose-response metaregression: circulating insulin-like growth factor I (IGF-I) and mortality. J Clin Endocrinol Metab 96:2912–2920. https://doi.org/10.1210/jc.2011-1377
Burkle A et al (2015) MARK-AGE biomarkers of ageing. Mech Ageing Dev 151:2–12. https://doi.org/10.1016/j.mad.2015.03.006
Cameron AR, Morrison VL, Levin D, Mohan M, Forteath C, Beall C, McNeilly AD, Balfour DJK, Savinko T, Wong AKF, Viollet B, Sakamoto K, Fagerholm SC, Foretz M, Lang CC, Rena G (2016) Anti-inflammatory effects of metformin irrespective of diabetes status. Circ Res 119:652–665. https://doi.org/10.1161/CIRCRESAHA.116.308445
Cappola AR, Xue QL, Ferrucci L, Guralnik JM, Volpato S, Fried LP (2003) Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J Clin Endocrinol Metab 88:2019–2025. https://doi.org/10.1210/jc.2002-021694
Cesari M et al (2003) Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation 108:2317–2322. https://doi.org/10.1161/01.CIR.0000097109.90783.FC
Chen BH et al (2016) DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany NY) 8:1844–1865. https://doi.org/10.18632/aging.101020
Cho K, Chung JY, Cho SK, Shin HW, Jang IJ, Park JW, Yu KS, Cho JY (2015) Antihyperglycemic mechanism of metformin occurs via the AMPK/LXRalpha/POMC pathway. Sci Rep 5:8145. https://doi.org/10.1038/srep08145
Chow SL, Maisel AS, Anand I, Bozkurt B, de Boer RA, Felker GM, Fonarow GC, Greenberg B, Januzzi JL Jr, Kiernan MS, Liu PP, Wang TJ, Yancy CW, Zile MR, American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology; Council on Basic Cardiovascular Sciences; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Epidemiology and Prevention; Council on Functional Genomics and Translational Biology; and Council on Quality of Care and Outcomes Research (2017) Role of biomarkers for the prevention, assessment, and management of heart failure: a scientific statement from the American Heart Association. Circulation 135:e1054–e1091. https://doi.org/10.1161/CIR.0000000000000490
Chung HK, Ryu D, Kim KS, Chang JY, Kim YK, Yi HS, Kang SG, Choi MJ, Lee SE, Jung SB, Ryu MJ, Kim SJ, Kweon GR, Kim H, Hwang JH, Lee CH, Lee SJ, Wall CE, Downes M, Evans RM, Auwerx J, Shong M (2017) Growth differentiation factor 15 is a myomitokine governing systemic energy homeostasis. J Cell Biol 216:149–165. https://doi.org/10.1083/jcb.201607110
Cohen AA, Legault V, Fuellen G, Fulop T, Fried LP, Ferrucci L (2017) The risks of biomarker-based epidemiology: associations of circulating calcium levels with age, mortality, and frailty vary substantially across populations. Exp Gerontol. https://doi.org/10.1016/j.exger.2017.07.011
Doi T, Shimada H, Makizako H, Tsutsumimoto K, Hotta R, Nakakubo S, Suzuki T (2016) Insulin-like growth factor-1 related to disability among older adults. J Gerontol A Biol Sci Med Sci 71:797–802. https://doi.org/10.1093/gerona/glv167
Dubowitz N et al (2014) Aging is associated with increased HbA1c levels, independently of glucose levels and insulin resistance, and also with decreased HbA1c diagnostic specificity. Diabet Med 31:927–935. https://doi.org/10.1111/dme.12459
Duca FA, Cote CD, Rasmussen BA, Zadeh-Tahmasebi M, Rutter GA, Filippi BM, Lam TK (2015) Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat Med 21:506–511. https://doi.org/10.1038/nm.3787
Engelfriet PM, Jansen EH, Picavet HS, Dolle ME (2013) Biochemical markers of aging for longitudinal studies in humans. Epidemiol Rev 35:132–151. https://doi.org/10.1093/epirev/mxs011
Espeland MA, Crimmins EM, Grossardt BR, Crandall JP, Gelfond JAL, Harris TB, Kritchevsky SB, Manson JAE, Robinson JG, Rocca WA, Temprosa M, Thomas F, Wallace R, Barzilai N, for the Multimorbidity Clinical Trials Consortium (2017) Clinical trials targeting aging and age-related multimorbidity. J Gerontol A Biol Sci Med Sci 72:355–361. https://doi.org/10.1093/gerona/glw220
Evans SJ, Sayers M, Mitnitski A, Rockwood K (2014) The risk of adverse outcomes in hospitalized older patients in relation to a frailty index based on a comprehensive geriatric assessment. Age Ageing 43:127–132. https://doi.org/10.1093/ageing/aft156
Fontana L, Klein S, Holloszy JO (2006) Long-term low-protein, low-calorie diet and endurance exercise modulate metabolic factors associated with cancer risk. Am J Clin Nutr 84:1456–1462
Fontana L, Partridge L, Longo VD (2010) Extending healthy life span--from yeast to humans. Science 328:321–326. https://doi.org/10.1126/science.1172539
Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B (2014) Metformin: from mechanisms of action to therapies. Cell Metab 20:953–966. https://doi.org/10.1016/j.cmet.2014.09.018
Foretz M, Hébrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B (2010) Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest 120:2355–2369. https://doi.org/10.1172/JCI40671
Foster MC et al (2013) Novel filtration markers as predictors of all-cause and cardiovascular mortality in US adults. Am J Kidney Dis 62:42–51. https://doi.org/10.1053/j.ajkd.2013.01.016
Fried LP et al (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56:M146–M156
Friedrich N et al (2009) Mortality and serum insulin-like growth factor (IGF)-I and IGF binding protein 3 concentrations. J Clin Endocrinol Metab 94:1732–1739. https://doi.org/10.1210/jc.2008-2138
Gerstein HC et al (2017) Growth differentiation factor 15 as a novel biomarker for metformin. Diabetes Care 40:280–283. https://doi.org/10.2337/dc16-1682
Giovannini S, onder G, Liperoti R, Russo A, Carter C, Capoluongo E, Pahor M, Bernabei R, Landi F (2011) Interleukin-6, C-reactive protein, and tumor necrosis factor-alpha as predictors of mortality in frail, community-living elderly individuals. J Am Geriatr Soc 59:1679–1685. https://doi.org/10.1111/j.1532-5415.2011.03570.x
Guevara-Aguirre J et al (2011) Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med 3:70ra13. https://doi.org/10.1126/scitranslmed.3001845
Hart A, Blackwell TL, Paudel ML, Taylor BC, Orwoll ES, Cawthon PM, Ensrud KE, for the Osteoporotic Fractures in Men (MrOS) Study Group (2017) Cystatin C and the risk of frailty and mortality in older men. J Gerontol A Biol Sci Med Sci 72:965–970. https://doi.org/10.1093/gerona/glw223
Hart A, Paudel ML, Taylor BC, Ishani A, Orwoll ES, Cawthon PM, Ensrud KE, Osteoporotic Fractures in Men Study Group (2013) Cystatin C and frailty in older men. J Am Geriatr Soc 61:1530–1536. https://doi.org/10.1111/jgs.12413
Harvie MN et al (2011) The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond) 35:714–727. https://doi.org/10.1038/ijo.2010.171
High KP, Kritchevsky SB (2015) Translational research in the fastest growing population: older adults. In: Wehling M (ed) Principles of translational science in medicine, 2nd edn. Academic Press; Elsevier Inc., Cambridge, pp 299–311. https://doi.org/10.1016/B978-0-12-800687-0.00031-1
Horvath S (2013) DNA methylation age of human tissues and cell types. Genome Biol 14:R115. https://doi.org/10.1186/gb-2013-14-10-r115
Horvath S et al (2016) An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biol 17:171. https://doi.org/10.1186/s13059-016-1030-0
Howlett SE, Rockwood MR, Mitnitski A, Rockwood K (2014) Standard laboratory tests to identify older adults at increased risk of death. BMC Med 12:171. https://doi.org/10.1186/s12916-014-0171-9
Hu D, Pawlikowska L, Kanaya A, Hsueh WC, Colbert L, Newman AB, Satterfield S, Rosen C, Cummings SR, Harris TB, Ziv E, for the Health, Aging, and Body Composition Study (2009) Serum insulin-like growth factor-1 binding proteins 1 and 2 and mortality in older adults: the health, aging, and body composition. Study J Am Geriatr Soc 57:1213–1218. https://doi.org/10.1111/j.1532-5415.2009.02318.x
Jiang J, Wen W, Sachdev PS (2016) Macrophage inhibitory cytokine-1/growth differentiation factor 15 as a marker of cognitive ageing and dementia. Curr Opin Psychiatry 29:181–186. https://doi.org/10.1097/YCO.0000000000000225
Jickling GC, Sharp FR (2015) Biomarker panels in ischemic stroke. Stroke 46:915–920. https://doi.org/10.1161/STROKEAHA.114.005604
Johnson JA, Simpson SH, Toth EL, Majumdar SR (2005) Reduced cardiovascular morbidity and mortality associated with metformin use in subjects with type 2 diabetes. Diabet Med 22:497–502. https://doi.org/10.1111/j.1464-5491.2005.01448.x
Justice J, Miller JD, Newman JC, Hashmi SK, Halter J, Austad SN, Barzilai N, Kirkland JL (2016) Frameworks for proof-of-concept clinical trials of interventions that target fundamental aging processes. J Gerontol A Biol Sci Med Sci 71:1415–1423. https://doi.org/10.1093/gerona/glw126
Jylhava J, Pedersen NL, Hagg S (2017) Biological age predictors. EBioMedicine 21:29–36. https://doi.org/10.1016/j.ebiom.2017.03.046
Kaplan RC, McGinn AP, Pollak MN, Kuller LH, Strickler HD, Rohan TE, Cappola AR, Xue XN, Psaty BM (2007) Association of total insulin-like growth factor-I, insulin-like growth factor binding protein-1 (IGFBP-1), and IGFBP-3 levels with incident coronary events and ischemic stroke. J Clin Endocrinol Metab 92:1319–1325. https://doi.org/10.1210/jc.2006-1631
Karasik D, Cheung CL, Zhou Y, Cupples LA, Kiel DP, Demissie S (2012) Genome-wide association of an integrated osteoporosis-related phenotype: is there evidence for pleiotropic genes? J Bone Miner Res 27:319–330. https://doi.org/10.1002/jbmr.563
Kempf T et al (2007) Growth-differentiation factor-15 improves risk stratification in ST-segment elevation myocardial infarction. Eur Heart J 28:2858–2865. https://doi.org/10.1093/eurheartj/ehm465
Kennedy BK et al (2014) Geroscience: linking aging to chronic disease. Cell 159:709–713. https://doi.org/10.1016/j.cell.2014.10.039
Kenyon C (2001) A conserved regulatory system for aging. Cell 105:165–168
Khan SS, Singer BD, Vaughan DE (2017) Molecular and physiological manifestations and measurement of aging in humans. Aging Cell 16:624–633. https://doi.org/10.1111/acel.12601
Kickstein E et al (2010) Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc Natl Acad Sci USA 107:21830–21835. https://doi.org/10.1073/pnas.0912793107
Kim KM et al (2018) SCAMP4 enhances the senescent cell secretome. Genes Dev 32:909–914. https://doi.org/10.1101/gad.313270.118
Kim S, Myers L, Wyckoff J, Cherry KE, Jazwinski SM (2017) The frailty index outperforms DNA methylation age and its derivatives as an indicator of biological age. Geroscience 39:83–92. https://doi.org/10.1007/s11357-017-9960-3
Kooy A, de Jager J, Lehert P, Bets D, Wulffele MG, Donker AJ, Stehouwer CD (2009) Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 169:616–625. https://doi.org/10.1001/archinternmed.2009.20
Landman GW, Kleefstra N, van Hateren KJ, Groenier KH, Gans RO, Bilo HJ (2010) Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care 33:322–326. https://doi.org/10.2337/dc09-1380
Lankeit M et al (2008) Growth differentiation factor-15 for prognostic assessment of patients with acute pulmonary embolism. Am J Respir Crit Care Med 177:1018–1025. https://doi.org/10.1164/rccm.200712-1786OC
Lara J, Cooper R, Nissan J, Ginty AT, Khaw KT, Deary IJ, Lord JM, Kuh D, Mathers JC (2015) A proposed panel of biomarkers of healthy ageing. BMC Med 13:222. https://doi.org/10.1186/s12916-015-0470-9
Laughlin GA, Barrett-Connor E, Criqui MH, Kritz-Silverstein D (2004) The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo study. J Clin Endocrinol Metab 89:114–120. https://doi.org/10.1210/jc.2003-030967
Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC (2011) Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer 11:20. https://doi.org/10.1186/1471-2407-11-20
Lee RY, Hench J, Ruvkun G (2001) Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the DAF-2 insulin-like signaling pathway. Curr Biol 11:1950–1957
Leng SX, Hung W, Cappola AR, Yu Q, Xue QL, Fried LP (2009) White blood cell counts, insulin-like growth factor-1 levels, and frailty in community-dwelling older women. J Gerontol A Biol Sci Med Sci 64:499–502. https://doi.org/10.1093/gerona/gln047
Lettieri-Barbato D, Giovannetti E, Aquilano K (2016) Effects of dietary restriction on adipose mass and biomarkers of healthy aging in human. Aging (Albany NY) 8:3341–3355. https://doi.org/10.18632/aging.101122
Levine ME, Hosgood HD, Chen B, Absher D, Assimes T, Horvath S (2015) DNA methylation age of blood predicts future onset of lung cancer in the women’s health initiative. Aging (Albany NY) 7:690–700. https://doi.org/10.18632/aging.100809
Levine ME et al (2018) An epigenetic biomarker of aging for lifespan and healthspan. Aging-Us 10:573–591. https://doi.org/10.18632/aging.101414
Li Q, Wang S, Milot E, Bergeron P, Ferrucci L, Fried LP, Cohen AA (2015) Homeostatic dysregulation proceeds in parallel in multiple physiological systems. Aging Cell 14:1103–1112. https://doi.org/10.1111/acel.12402
Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM (2009) New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 32:1620–1625. https://doi.org/10.2337/dc08-2175
Lio D et al (2003) Inflammation, genetics, and longevity: further studies on the protective effects in men of IL-10-1082 promoter SNP and its interaction with TNF-alpha -308 promoter SNP. J Med Genet 40:296–299
Liu B, Fan Z, Edgerton SM, Yang X, Lind SE, Thor AD (2011) Potent anti-proliferative effects of metformin on trastuzumab-resistant breast cancer cells via inhibition of erbB2/IGF-1 receptor interactions. Cell Cycle 10:2959–2966
Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C, Curiel TJ, de Cabo R, Franceschi C, Gems D, Ingram DK, Johnson TE, Kennedy BK, Kenyon C, Klein S, Kopchick JJ, Lepperdinger G, Madeo F, Mirisola MG, Mitchell JR, Passarino G, Rudolph KL, Sedivy JM, Shadel GS, Sinclair DA, Spindler SR, Suh Y, Vijg J, Vinciguerra M, Fontana L (2015) Interventions to slow aging in humans: are we ready? Aging Cell 14:497–510. https://doi.org/10.1111/acel.12338
Longo VD, Finch CE (2003) Evolutionary medicine: from dwarf model systems to healthy centenarians? Science 299:1342–1346. https://doi.org/10.1126/science.1077991
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153:1194–1217. https://doi.org/10.1016/j.cell.2013.05.039
Lu J et al (2015) Activation of AMPK by metformin inhibits TGF-beta-induced collagen production in mouse renal fibroblasts. Life Sci 127:59–65. https://doi.org/10.1016/j.lfs.2015.01.042
Luchsinger JA, Perez T, Chang H, Mehta P, Steffener J, Pradabhan G, Ichise M, Manly J, Devanand DP, Bagiella E (2016) Metformin in amnestic mild cognitive impairment: results of a pilot randomized placebo controlled clinical trial. J Alzheimers Dis 51:501–514. https://doi.org/10.3233/JAD-150493
Maggio M et al (2007) Relationship between low levels of anabolic hormones and 6-year mortality in older men: the aging in the chianti area (InCHIANTI) study. Arch Intern Med 167:2249–2254. https://doi.org/10.1001/archinte.167.20.2249
Mao K et al (2018) Late-life targeting of the IGF-1 receptor improves healthspan and lifespan in female mice. Nat Commun 9:2394. https://doi.org/10.1038/s41467-018-04805-5
Marioni RE, Shah S, McRae AF, Chen BH, Colicino E, Harris SE, Gibson J, Henders AK, Redmond P, Cox SR, Pattie A, Corley J, Murphy L, Martin NG, Montgomery GW, Feinberg AP, Fallin M, Multhaup ML, Jaffe AE, Joehanes R, Schwartz J, Just AC, Lunetta KL, Murabito JM, Starr JM, Horvath S, Baccarelli AA, Levy D, Visscher PM, Wray NR, Deary IJ (2015a) DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol 16:25. https://doi.org/10.1186/s13059-015-0584-6
Marioni RE et al (2015b) The epigenetic clock is correlated with physical and cognitive fitness in the Lothian birth cohort 1936. Int J Epidemiol 44:1388–1396. https://doi.org/10.1093/ije/dyu277
Marti CN et al (2014) Soluble tumor necrosis factor receptors and heart failure risk in older adults: health, aging, and body composition (Health ABC) study. Circ Heart Fail 7:5–11. https://doi.org/10.1161/CIRCHEARTFAILURE.113.000344
Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, Schwab M, Pollak M, Zhang Y, Yu Y, Becker KG, Bohr VA, Ingram DK, Sinclair DA, Wolf NS, Spindler SR, Bernier M, de Cabo R (2013) Metformin improves healthspan and lifespan in mice. Nat Commun 4:2192. https://doi.org/10.1038/ncomms3192
Martin-Ruiz C et al (2011) Assessment of a large panel of candidate biomarkers of ageing in the Newcastle 85+ study. Mech Ageing Dev 132:496–502. https://doi.org/10.1016/j.mad.2011.08.001
Masson S et al (2006) Direct comparison of B-type natriuretic peptide (BNP) and amino-terminal proBNP in a large population of patients with chronic and symptomatic heart failure: the valsartan heart failure (Val-HeFT) data. Clin Chem 52:1528–1538. https://doi.org/10.1373/clinchem.2006.069575
Masternak MM, Bartke A (2012) Growth hormone, inflammation and aging. Pathobiol Aging Age Relat Dis 2:2. https://doi.org/10.3402/pba.v2i0.17293
McCabe EL, Larson MG, Lunetta KL, Newman AB, Cheng S, Murabito JM (2016) Association of an index of healthy aging with incident cardiovascular disease and mortality in a community-based sample of older adults. J Gerontol A Biol Sci Med Sci 71:1695–1701. https://doi.org/10.1093/gerona/glw077
McKay HS et al (2017) Multiplex assay reliability and long-term intra-individual variation of serologic inflammatory biomarkers. Cytokine 90:185–192. https://doi.org/10.1016/j.cyto.2016.09.018
Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, Cesari M, Nourhashemi F (2013) Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc 14:877–882. https://doi.org/10.1016/j.jamda.2013.05.009
Mitnitski A, Collerton J, Martin-Ruiz C, Jagger C, von Zglinicki T, Rockwood K, Kirkwood TB (2015) Age-related frailty and its association with biological markers of ageing. BMC Med 13:161. https://doi.org/10.1186/s12916-015-0400-x
Mitnitski A, Rockwood K (2015) Aging as a process of deficit accumulation: its utility and origin. Interdiscip Top Gerontol 40:85–98. https://doi.org/10.1159/000364933
Mitnitski A, Song X, Rockwood K (2013) Assessing biological aging: the origin of deficit accumulation. Biogerontology 14:709–717. https://doi.org/10.1007/s10522-013-9446-3
Mitnitski AB, Mogilner AJ, MacKnight C, Rockwood K (2002) The mortality rate as a function of accumulated deficits in a frailty index. Mech Ageing Dev 123:1457–1460
Moiseeva O et al (2013) Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-kappaB activation. Aging Cell 12:489–498. https://doi.org/10.1111/acel.12075
Nair V, Sreevalsan S, Basha R, Abdelrahim M, Abudayyeh A, Rodrigues Hoffman A, Safe S (2014) Mechanism of metformin-dependent inhibition of mammalian target of rapamycin (mTOR) and Ras activity in pancreatic cancer: role of specificity protein (Sp) transcription factors. J Biol Chem 289:27692–27701. https://doi.org/10.1074/jbc.M114.592576
Newman AB, Boudreau RM, Naydeck BL, Fried LF, Harris TB (2008) A physiologic index of comorbidity: relationship to mortality and disability. J Gerontol A Biol Sci Med Sci 63:603–609
Newman AB, Sanders JL, Kizer JR, Boudreau RM, Odden MC, Zeki Al Hazzouri A, Arnold AM (2016a) Trajectories of function and biomarkers with age: the CHS all stars study. Int J Epidemiol 45:1135–1145. https://doi.org/10.1093/ije/dyw092
Newman JC, Milman S, Hashmi SK, Austad SN, Kirkland JL, Halter JB, Barzilai N (2016b) Strategies and challenges in clinical trials targeting human aging. J Gerontol A Biol Sci Med Sci 71:1424–1434. https://doi.org/10.1093/gerona/glw149
Ng TP, Feng L, Yap KB, Lee TS, Tan CH, Winblad B (2014) Long-term metformin usage and cognitive function among older adults with diabetes. J Alzheimers Dis 41:61–68. https://doi.org/10.3233/JAD-131901
O'Connell MDL et al (2018) Mortality in relation to changes in a healthy aging index: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. https://doi.org/10.1093/gerona/gly114
Odden MC et al (2010) Age and cystatin C in healthy adults: a collaborative study. Nephrol Dial Transplant 25:463–469. https://doi.org/10.1093/ndt/gfp474
Omland T et al (2007) Prognostic value of B-type natriuretic peptides in patients with stable coronary artery disease: the PEACE trial. J Am Coll Cardiol 50:205–214. https://doi.org/10.1016/j.jacc.2007.03.038
Palta P, Huang ES, Kalyani RR, Golden SH, Yeh HC (2017) Hemoglobin A1c and mortality in older adults with and without diabetes: results from the national health and nutrition examination surveys (1988-2011). Diabetes Care 40:453–460. https://doi.org/10.2337/dci16-0042
Pani LN et al (2008) Effect of aging on A1C levels in individuals without diabetes: evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001-2004. Diabetes Care 31:1991–1996. https://doi.org/10.2337/dc08-0577
Penninx BW et al (2004) Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc 52:1105–1113. https://doi.org/10.1111/j.1532-5415.2004.52308.x
Pijl H, Langendonk JG, Burggraaf J, Frolich M, Cohen AF, Veldhuis JD, Meinders AE (2001) Altered neuroregulation of GH secretion in viscerally obese premenopausal women. J Clin Endocrinol Metab 86:5509–5515. https://doi.org/10.1210/jcem.86.11.8061
Quach A et al (2017) Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging (Albany NY) 9:419–446. https://doi.org/10.18632/aging.101168
Reuben DB, Cheh AI, Harris TB, Ferrucci L, Rowe JW, Tracy RP, Seeman TE (2002) Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J Am Geriatr Soc 50:638–644
Rincon M, Rudin E, Barzilai N (2005) The insulin/IGF-1 signaling in mammals and its relevance to human longevity. Exp Gerontol 40:873–877. https://doi.org/10.1016/j.exger.2005.06.014
Rochon J et al (2011) Design and conduct of the CALERIE study: comprehensive assessment of the long-term effects of reducing intake of energy. J Gerontol A Biol Sci Med Sci 66:97–108. https://doi.org/10.1093/gerona/glq168
Rodgers BD (2016) The immateriality of circulating GDF11. Circ Res 118:1472–1474. https://doi.org/10.1161/Circresaha.116.308478
Rodgers BD, Eldridge JA (2015) Reduced circulating GDF11 is unlikely responsible for age-dependent changes in mouse heart, muscle, and brain. Endocrinology 156:3885–3888. https://doi.org/10.1210/en.2015-1628
Roubenoff R et al (2003) Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham heart study. Am J Med 115:429–435
Roussel R et al (2010) Metformin use and mortality among patients with diabetes and atherothrombosis. Arch Intern Med 170:1892–1899. https://doi.org/10.1001/archinternmed.2010.409
Saisho Y (2015) Metformin and inflammation: its potential beyond glucose-lowering effect. Endocr Metab Immune Disord Drug Targets 15:196–205
Sanders JL et al (2018) Association of biomarker and physiologic indices with mortality in older adults: cardiovascular health study. J Gerontol A Biol Sci Med Sci. https://doi.org/10.1093/gerona/gly075
Sanders JL, Boudreau RM, Newman AB (2012a) Understanding the aging process using epidemiologic approaches. In: The epidemiology of aging. Springer, Dordrecht, pp 187–214
Sanders JL, Boudreau RM, Penninx BW, Simonsick EM, Kritchevsky SB, Satterfield S, Harris TB, Bauer DC, Newman AB, for the Health ABC Study (2012b) Association of a modified physiologic index with mortality and incident disability: the health, aging, and body composition study. J Gerontol A Biol Sci Med Sci 67:1439–1446. https://doi.org/10.1093/gerona/gls123
Sanders JL et al (2014) Heritability of and mortality prediction with a longevity phenotype: the healthy aging index. J Gerontol A Biol Sci Med Sci 69:479–485. https://doi.org/10.1093/gerona/glt117
Sanders JL, Newman AB (2013) Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither? Epidemiol Rev 35:112–131. https://doi.org/10.1093/epirev/mxs008
Sarnak MJ et al (2008) Cystatin C and aging success. Arch Intern Med 168:147–153. https://doi.org/10.1001/archinternmed.2007.40
Saydah S, Graubard B, Ballard-Barbash R, Berrigan D (2007) Insulin-like growth factors and subsequent risk of mortality in the United States. Am J Epidemiol 166:518–526. https://doi.org/10.1093/aje/kwm124
Schafer MJ et al (2016) Quantification of GDF11 and myostatin in human aging and cardiovascular disease. Cell Metabolism 23:1207–1215. https://doi.org/10.1016/j.cmet.2016.05.023
Schramm TK et al (2011) Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. Eur Heart J 32:1900–1908. https://doi.org/10.1093/eurheartj/ehr077
Sebastiani P et al (2016) Age and sex distributions of age-related biomarker values in healthy older adults from the long life family study. J Am Geriatr Soc 64:e189–e194. https://doi.org/10.1111/jgs.14522
Sebastiani P, Thyagarajan B, Sun F, Schupf N, Newman AB, Montano M, Perls TT (2017) Biomarker signatures of aging. Aging Cell 16:329–338. https://doi.org/10.1111/acel.12557
Shaver LN, Beavers DP, Kiel J, Kritchevsky SB, Beavers KM (2018) Effect of intentional weight loss on mortality biomarkers in older adults with obesity. J Gerontol A Med Sci. https://doi.org/10.1093/gerona/gly192
Shlipak MG, Wassel Fyr CL, Chertow GM, Harris TB, Kritchevsky SB, Tylavsky FA, Satterfield S, Cummings SR, Newman AB, Fried LF (2006) Cystatin C and mortality risk in the elderly: the health, aging, and body composition study. J Am Soc Nephrol 17:254–261. https://doi.org/10.1681/ASN.2005050545
Sierra F (2016a) The emergence of geroscience as an interdisciplinary approach to the enhancement of health span and life span. Cold Spring Harb Perspect Med 6:a025163. https://doi.org/10.1101/cshperspect.a025163
Sierra F (2016b) Moving geroscience into uncharted waters. J Gerontol A Biol Sci Med Sci 71:1385–1387. https://doi.org/10.1093/gerona/glw087
Smith DL Jr, Elam CF Jr, Mattison JA, Lane MA, Roth GS, Ingram DK, Allison DB (2010) Metformin supplementation and life span in Fischer-344 rats. J Gerontol A Biol Sci Med Sci 65:468–474. https://doi.org/10.1093/gerona/glq033
Sprott RL (1988) Biomarkers of aging. Exp Gerontol 23:1–3
Sprott RL (2010) Biomarkers of aging and disease: introduction and definitions. Exp Gerontol 45:2–4. https://doi.org/10.1016/j.exger.2009.07.008
Steuerman R, Shevah O, Laron Z (2011) Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Eur J Endocrinol 164:485–489. https://doi.org/10.1530/EJE-10-0859
Stork S et al (2006) Prediction of mortality risk in the elderly. Am J Med 119:519–525. https://doi.org/10.1016/j.amjmed.2005.10.062
Strong R et al (2016) Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an alpha-glucosidase inhibitor or a Nrf2-inducer. Aging Cell 15:872–884. https://doi.org/10.1111/acel.12496
Svensson-Farbom P et al (2014) Cystatin C identifies cardiovascular risk better than creatinine-based estimates of glomerular filtration in middle-aged individuals without a history of cardiovascular disease. J Intern Med 275:506–521. https://doi.org/10.1111/joim.12169
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Task Force for the Universal Definition of Myocardial I (2012) Third universal definition of myocardial infarction. Nat Rev Cardiol 9:620–633. https://doi.org/10.1038/nrcardio.2012.122
van der Spoel E et al (2015) Association analysis of insulin-like growth factor-1 axis parameters with survival and functional status in nonagenarians of the Leiden longevity study. Aging (Albany NY) 7:956–963. https://doi.org/10.18632/aging.100841
Varadhan R et al (2014) Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. J Gerontol a-Biol 69:165–173. https://doi.org/10.1093/gerona/glt023
Vinel C et al (2018) The exerkine apelin reverses age-associated sarcopenia. Nat Med. https://doi.org/10.1038/s41591-018-0131-6
von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27:339–344
Wagner KH, Cameron-Smith D, Wessner B, Franzke B (2016) Biomarkers of aging: from function to molecular biology. Nutrients 8:8. https://doi.org/10.3390/nu8060338
Wang T et al (2017) Epigenetic aging signatures in mice livers are slowed by dwarfism, calorie restriction and rapamycin treatment. Genome Biol 18:57. https://doi.org/10.1186/s13059-017-1186-2
Welsh JB et al (2003) Large-scale delineation of secreted protein biomarkers overexpressed in cancer tissue and serum. Proc Natl Acad Sci U S A 100:3410–3415. https://doi.org/10.1073/pnas.0530278100
Wiklund FE et al (2010) Macrophage inhibitory cytokine-1 (MIC-1/GDF15): a new marker of all-cause mortality. Aging Cell 9:1057–1064. https://doi.org/10.1111/j.1474-9726.2010.00629.x
Wu C, Smit E, Sanders JL, Newman AB, Odden MC (2017) A modified healthy aging index and its association with mortality: the National Health and Nutrition Examination Survey, 1999-2002. J Gerontol A Biol Sci Med Sci 72:1437–1444. https://doi.org/10.1093/gerona/glw334
Wu CK, Chang MH, Lin JW, Caffrey JL, Lin YS (2011) Renal-related biomarkers and long-term mortality in the US subjects with different coronary risks. Atherosclerosis 216:226–236. https://doi.org/10.1016/j.atherosclerosis.2011.01.046
Xia X, Chen W, McDermott J, Han JJ (2017) Molecular and phenotypic biomarkers of aging. F1000Res 6:860. https://doi.org/10.12688/f1000research.10692.1
Zheng Z et al (2012) Sirtuin 1-mediated cellular metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and therapeutic effects of metformin. Diabetes 61:217–228. https://doi.org/10.2337/db11-0416
Funding
This study received financial support from the American Federation for Aging Research (AFAR); the Glenn Center for the Biology of Human Aging (Paul Glenn Foundation for Medical Research); National Institutes of Health: K01 AG059837-01 (JNJ), P30 AG021332 (SKB, JNJ); R01 AG048023, R01 AG052608, R35 GM124922 (GAK); P30 AG038072 (NB); R01 AG023629 (ABN), and supported in part by the Intramural Research Program of the National Institute on Aging, National Institute of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
JNJ, NB, JB, JD, MAE, LF, SBK, SM, ABN, MP, and GAK report no conflicts of interest to this work. VRA was the MedStar Health Research Institute’s principal clinical trial investigator for studies involving Elcelyx (delayed release metformin).
Electronic supplementary material
ESM 1
(DOCX 652 kb)
About this article
Cite this article
Justice, J.N., Ferrucci, L., Newman, A.B. et al. A framework for selection of blood-based biomarkers for geroscience-guided clinical trials: report from the TAME Biomarkers Workgroup. GeroScience 40, 419–436 (2018). https://doi.org/10.1007/s11357-018-0042-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-018-0042-y