Abstract
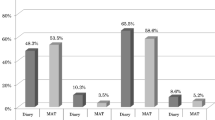
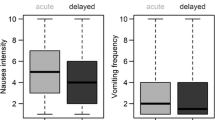
Background International guidelines are tools enabling physicians to incorporate the latest evidence based clinical information into practice. Objective This study aimed to evaluate the impact of antiemetic guidelines adherence on the incidence of chemotherapy-induced nausea and vomiting (CINV) and patient quality of life. Setting Marmara University Pendik Training and Research Hospital chemotherapy unit, Istanbul, Turkey. Method The study included 100 chemotherapy naive patients. Antiemetic prescribing patterns and their consistency with MASCC/ESMO 2014 guidelines were assessed. Patients recorded incidences of vomiting in a daily dairy and described their nausea using a 7-item Likert Scale. The incidence of CINV was recorded over five days. To assess the patient's quality of life, a modified Turkish version of the Functional Living Index-Emesis (FLIE) questionnaire was administered before and after receiving chemotherapy. A questionnaire on the existence and severity of side effects was developed and administered. Main outcome measures Incidence of side effects on CINV and quality of life according to the FLIE. Results The primary outcome revealed differences in complete control (no emetic episodes, rescue therapy or nausea), FLIE scores and side effects. Guidelines consistency was observed more with acute (A) than with delayed (D) prevention of CINV, with significant differences in complete control between the guideline adherent group (GAG) and the guideline nonadherent group (GNG). Significant differences in the FLIE score were noticed between GAG(D) and GNG(D), and GNG(D) had a higher incidence of diarrhoea, headache, swallowing difficulties and dark-coloured stool. Conclusion Consistency with guidelines resulted in significant reduction in the incidence of both cute and delayed CINV and other side effects, and with improvement of the patient quality of life.


Similar content being viewed by others
References
Herrstedt J. Nausea and emesis: Still an unsolved problem in cancer patients? Support Care Cancer. 2002;10:85–7.
Tina Shih Y, Xu Y, Elting L. Costs of uncontrolled chemotherapy-induced nausea and vomiting among working-age cancer patients receiving highly or moderately emetogenic chemotherapy. Cancer. 2007;110:678–85.
Lindley CM, Hirsch JD. Nausea and vomiting and cancer patients’ quality of life: a discussion of Professor Selby’s paper. Br J Cancer. 1992;19:S26–9.
Bloechl-Daum B. Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006;24:4472–8.
Ballatori E, Roila F, Ruggeri B, Betti M, Sarti S, Soru G, et al. The impact of chemotherapy-induced nausea and vomiting on health-related quality of life. Support Care Cancer. 2006;15:179–85.
Cohen L, de Moor C, Eisenberg P, Ming E, Hu H. Chemotherapy-induced nausea and vomiting incidence and impact on patient quality of life at community oncology settings. Support Care Cancer. 2006;15:497–503.
Fernandez-Ortega P, Caloto M, Chirveches E, Marquilles R, Francisco J, Quesada A, et al. Chemotherapy-induced nausea and vomiting in clinical practice: impact on patients’ quality of life. Support Care Cancer. 2012;20:3141–8.
Jordan NS, Schauer PK, Schauer A, Nightingale C, Golub G, Martin RS, et al. The effect of administration rate on cisplatin-induced emesis. J Clin Oncol. 1985;3:559–61.
Basch E, Hesketh P, Kris M, Prestrud A, Temin S, Lyman G. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. JOP. 2011;7:395–8.
Roila F, Herrstedt J, Aapro M, Gralla R, Einhorn L, Ballatori E, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21:232–43.
Morrow GR, Roscoe JA, Hickok JT, Stern RM, Pierce HI, King DB, et al. Initial control of chemotherapy-induced nausea and vomiting in patient quality of life. Oncology (Williston Park). 1998;12:32–7.
Kirkova J, Rybicki L, Walsh D, Aktas A. Symptom prevalence in advanced cancer: age, gender, and performance status interactions. Am J Hosp Palliat Med. 2011;29:139–45.
Kaiser R. Patient-tailored antiemetic treatment with 5-hydroxytryptamine type 3 receptor antagonists according to cytochrome P-450 2D6 genotypes. J Clin Oncol. 2002;20:2805–11.
Tremblay P. Variations in the 5-hydroxytryptamine type 3B receptor gene as predictors of the efficacy of antiemetic treatment in cancer patients. J Clin Oncol. 2003;21:2147–55.
Morrow G, Roscoe J, Kirshner J, Hynes H, Rosenbluth R. Anticipatory nausea and vomiting in the era of 5-HT3 antiemetics. Support Care Cancer. 1998;6:244–7.
Fallowfield L. Behavioural interventions and psychological aspects of care during chemotherapy. Eur J Cancer. 1992;28:S39–41.
Morrow G. The effect of a susceptibility to motion sickness on the side effects of cancer chemotherapy. Cancer. 1985;55:2766–70.
Jordan K, Gralla R, Jahn F, Molassiotis A. International antiemetic guidelines on chemotherapy induced nausea and vomiting (CINV): content and implementation in daily routine practice. Eur J Pharmacol. 2014;722:197–202.
Peterson C, Hursti T, Barjeson S, Ãvall-Lundgvist E, Fredrikson M, Farst C, et al. Single high-dose dexamethasone improves the effect of ondansetron on acute chemotherapy-induced nausea and vomiting but impairs the control of delayed symptoms. Support Care Cancer. 1996;4:440–6.
Gralla R. Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol. 2003;14:1570–7.
Aksu G, Dolasik I, Ensaroglu F, Sener S, Aydin F, Temiz S, et al. Evaluation of the efficacy of aprepitant on the prevention of chemotherapy-induced nausea and vomiting and quality of life with functional living index emesis. Balk Med J. 2013;30:64–7.
Berger AM, Clark-Snow RA. Nausea and vomiting. In: DeVita Jr VT, Hellman S, Rosenberg SA, editors. Cancer: principles & practice of oncology. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2004.
Italian Group for Antiemetic Research. Double-blind, dose-finding study of four intravenous doses of dexamethasone in the prevention of cisplatin-induced acute emesis. J Clin Oncol. 1998;16:2937–42.
McCrea J. Effects of the neurokinin1 receptor antagonist aprepitant on the pharmacokinetics of dexamethasone and methylprednisolone. Clin Pharmacol Ther. 2003;74:17–24.
Hesketh P. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin-the aprepitant protocol 052 study group. J Clin Oncol. 2003;21:4112–9.
Poli-Bigelli S, Rodrigues-Pereira J, Carides A, Julie Ma G, Eldridge K, Hipple A, et al. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Cancer. 2003;97:3090–8.
Warr D. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol. 2004;23:2822–30.
Olver I, Paska W, Depierre A, Seitz J, Stewart D, Goedhals L, et al. A multicentre, double-blind study comparing placebo, ondansetron and ondansetron plus dexamethasone for the control of cisplatin-induced delayed emesis. Ann Oncol. 1996;7:945–52.
Navari RM, Madajewicz S, Anderson N, Tchekmedyian NS, Whaley W, Garewal H, et al. Oral ondansetron for the control of cisplatin-induced delayed emesis: a large, multicenter, double-blind, randomized comparative trial of ondansetron versus placebo. J Clin Oncol. 1995;13:2408–16.
Goedhals L, Heron J, Kleisbauer J, Pagani O, Sessa C. Control of delayed nausea and vomiting with granisetron plus dexamethasone or dexamethasone alone in patients receiving highly comparative study. Ann Oncol. 1998;9:661–6.
Geling O. Should 5-hydroxytryptamine-3 receptor antagonists be administered beyond 24 hours after chemotherapy to prevent delayed emesis? systematic re-evaluation of clinical evidence and drug cost implications. J Clin Oncol. 2005;23:1289–94.
Aapro M. A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol. 2006;17:1441–9.
Saito M, Aogi K, Sekine I, Yoshizawa H, Yanagita Y, Sakai H, et al. Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol. 2009;10:115–24.
Chan A, Shih V, Chew L. Evolving roles of oncology pharmacists in Singapore: a survey on prescribing patterns of antiemetics for chemotherapy induced nausea and vomiting (CINV) at a cancer centre. J Oncol Pharm Pract. 2008;14:23–9.
Eisenberg P, Figueroa-Vadillo J, Zamora R, Charu V, Hajdenberg J, Cartmell A, et al. Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist. Cancer. 2003;98:2473–82.
Likun Z, Xiang J, Yi B, Xin D, Tao Z. A systematic review and meta-analysis of intravenous palonosetron in the prevention of chemotherapy-induced nausea and vomiting in adults. Oncologist. 2011;16:207–16.
Fujii H, Iihara H, Ishihara M, Takahashi T, Yoshida K, Itoh Y. Improvement of adherence to guidelines for antiemetic medication enhances emetic control in patients with colorectal cancer receiving chemotherapy of moderate emetic risk. Anticancer Res. 2013;33:5549–56.
Almazrou S, Alnaim L. Evaluation of adherence to chemotherapy-induced nausea and vomiting guidelines. An Observational Study. J Cancer Ther. 2012;3:613–20.
Aapro M, Molassiotis A, Dicato M, Pelaez I, Rodriguez-Lescure A, Pastorelli D, et al. The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): the Pan European Emesis Registry (PEER). Ann Oncol. 2012;23:1986–92.
Gilmore J, Peacock N, Gu A, Szabo S, Rammage M, Sharpe J, et al. Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community oncology practice: INSPIRE Study. JOP. 2013;10:68–74.
Goodin S. 5-HT3-receptor antagonists for the treatment of nausea and vomiting: a reappraisal of their side-effect profile. Oncologist. 2002;7:424–36.
Meyerowitz B, Sparks F, Spears I. Adjuvant chemotherapy for breast carcinoma: psychosocial implications. Cancer. 1979;43:1613–8.
Acknowledgements
The authors are grateful to Merve Sarı who assisted in identifying patients.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
Functional Living Index-EMESIS (English Version)
The Functional Living Index-Emesis (FLIE) is an emesis-specific questionnaire with 18 questions answered on a scale of 1–7. The FLIE can be divided into nausea- and vomiting-specific questions. A FLIE nausea summary score was determined by totalling the responses to the first 9 questions, and a FLIE vomiting summary score by totalling the responses to the last 9 questions. The FLIE includes 1 question that specifically asks about nausea and 1 that specifically asks about vomiting; the other questions assess how nausea and vomiting are interfering with the patient’s daily life. A lower score represents increased symptoms or worse quality of life (QoL). Patients completed the FLIE at baseline and at days 5 after completion of first chemotherapy cycle.


Fonksiyonel Yaşam ölçeği-Emesis(Turkish Version)
Fonksiyonel Yaşam Endeksi-Kusma (FlIE) 1–7 bir ölçekte cevap 18 soru ile bir kusma özgü bir ankettir. FLIE bulantı- ve kusma-spesifik sorulara ayrılabilir. Bir FlIE bulantı özeti skoru ilk 9 soruların yanıtları toplam belirlenir ve son 9 soruların yanıtları toplam bir FlIE kusma özeti puan aldı. FlIE özellikle bulantı ve kusma özellikle sorar yaklaşık 1 sorar 1 soru içerir; Diğer sorular mide bulantısı ve kusma hastanın günlük yaşam müdahale nasıl değerlendirmek. Düşük puan artan belirtiler ya da yaşam (YK) daha kötü kalitesini temsil eder. Hastalar ilk kemoterapi devrinin tamamlanmasından sonra 5 başlangıçta ve gün FlIE tamamladı.


Rights and permissions
About this article
Cite this article
Abunahlah, N., Sancar, M., Dane, F. et al. Impact of adherence to antiemetic guidelines on the incidence of chemotherapy-induced nausea and vomiting and quality of life. Int J Clin Pharm 38, 1464–1476 (2016). https://doi.org/10.1007/s11096-016-0393-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-016-0393-3