Abstract
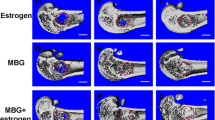
The most common complication for patients with postmenopausal osteoporosis is bone-related defects and fractures. While routine medication has a high probability of undesirable side effects, new approaches have aimed to develop regeneration procedures that stimulate new bone formation while reversing bone loss. Recently, we have synthesized a new hybrid CaP/silk scaffold with a CaP-phase distribution and pore architecture better suited to facilitate cell differentiation and bone formation. The aim of the present study was to compare the involved remodeling process and therapeutic effect of porous CaP/silk composite scaffolds upon local implantation into osteoporotic defects. Wistar rats were used to induce postmenopausal osteoporotic model by bilateral ovariectomy. The pure silk and hybrid CaP/silk scaffolds were implanted into critical sized defects created in distal femoral epiphysis. After 14 and 28 days, the in vivo osteogenetic efficiency was evaluated by μCT analysis, hematoxylin and eosin staining, Safranin O staining, tartrate-resistant acid phosphatase staining, and immunohistochemical assessment. Animals with or without critical-sized defects were used as drill or blank controls, respectively. The osteoporotic defect model was well established with significantly decreased μCT parameters of BV/TV, Tb.N and increased Tb.Sp, porosity, combined with changes in histological observations. During the healing process, the critical-sized drill control defects failed to regenerate appreciable bone tissue, while more significantly increased bone formation and mineralization with dynamic scaffold degradation and decreased osteoclastic bone resorption could be detected within defects with hybrid CaP/silk scaffolds compared to pure silk scaffolds.









Similar content being viewed by others
References
Tontonoz P, Pei LM. Fat’s loss is bone’s gain. J Clin Investig. 2004;113:805–6.
Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–14.
Cooper C, Campion G, Melton LJ 3rd. Hip fractures in the elderly: a world-wide projection. Osteoporos Int. 1992;2:285–9.
Teitelbaum SL. Stem cells and osteoporosis therapy. Cell Stem Cell. 2010;7:553–4.
Moazzaz P, Gupta MC, Gilotra MM, Gilotra MN, Maitra S, et al. Estrogen-dependent actions of bone morphogenetic protein-7 on spine fusion in rats. Spine (Phila Pa 1976). 2005;30:1706–11.
Hao YJ, Zhang G, Wang YS, Qin L, Hung WY, et al. Changes of microstructure and mineralized tissue in the middle and late phase of osteoporotic fracture healing in rats. Bone. 2007;41:631–8.
Namkung-Matthai H, Appleyard R, Jansen J, Hao Lin J, Maastricht S, et al. Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone. 2001;28:80–6.
Silva BC, Bilezikian JP. New approaches to the treatment of osteoporosis. Annu Rev Med. 2011;62:307–22.
Kaplan D, Adams WW, Farmer B, Viney C. Silk—biology, structure, properties, and genetics. ACS Symp Ser. 1994;544:2–16.
Kaplan DL, Wang YZ, Kim HJ, Vunjak-Novakovic G. Stem cell-based tissue engineering with silk biomaterials. Biomaterials. 2006;27:6064–82.
Kaplan DL, Vepari C. Silk as a biomaterial. Prog Polym Sci. 2007;32:991–1007.
Wang Y, Rudym DD, Walsh A, Abrahamsen L, Kim HJ, et al. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials. 2008;29:3415–28.
Meinel L, Hofmann S, Karageorgiou V, Kirker-Head C, McCool J, et al. The inflammatory responses to silk films in vitro and in vivo. Biomaterials. 2005;26:147–55.
Zhao J, Zhang Z, Wang S, Sun X, Zhang X, et al. Apatite-coated silk fibroin scaffolds to healing mandibular border defects in canines. Bone. 2009;45:517–27.
Costantino PD, Chaplin JM, Wolpoe ME, Catalano PJ, Sen C, et al. Applications of fast-setting hydroxyapatite cement: cranioplasty. Otolaryngol Head Neck Surg. 2000;123:409–12.
Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005;36(Suppl 3):S20–7.
Goyenvalle E, Guyen NJ, Aguado E, Passuti N, Daculsi G. Bilayered calcium phosphate coating to promote osseointegration of a femoral stem prosthesis. J Mater Sci Mater Med. 2003;14:219–27.
Barrere F, van Blitterswijk CA, de Groot K. Bone regeneration: molecular and cellular interactions with calcium phosphate ceramics. Int J Nanomed. 2006;1:317–32.
Zhang Y, Wu C, Friis T, Xiao Y. The osteogenic properties of CaP/silk composite scaffolds. Biomaterials. 2010;31:2848–56.
Sofia S, McCarthy MB, Gronowicz G, Kaplan DL. Functionalized silk-based biomaterials for bone formation. J Biomed Mater Res. 2001;54:139–48.
Lima I, Farias MLF, Percegoni N, Rosenthal D, de Assis JT, et al. Micro imaging analysis for osteoporosis assessment. Spectrochim Acta Part B At Spectrosc. 2010;65:253–7.
Mankani MH, Kuznetsov SA, Avila NA, Kingman A, Robey PG. Bone formation in transplants of human bone marrow stromal cells and hydroxyapatite-tricalcium phosphate: prediction with quantitative CT in mice. Radiology. 2004;230:369–76.
Hutmacher DW, Sawyer AA, Song SJ, Susanto E, Chuan P, et al. The stimulation of healing within a rat calvarial defect by mPCL-TCP/collagen scaffolds loaded with rhBMP-2. Biomaterials. 2009;30:2479–88.
Ebneter A, Casson RJ, Wood JP, Chidlow G. Microglial activation in the visual pathway in experimental glaucoma: spatiotemporal characterization and correlation with axonal injury. Investig Ophthalmol Vis Sci. 2010;51:6448–60.
Fernandes JC, Wang H, Jreyssaty C, Benderdour M, Lavigne P, et al. Bone-protective effects of nonviral gene therapy with folate-chitosan DNA nanoparticle containing interleukin-1 receptor antagonist gene in rats with adjuvant-induced arthritis. Mol Ther. 2008;16:1243–51.
Kawaguchi H, Akune T, Ohba S, Kamekura S, Yamaguchi M, et al. PPAR gamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Investig. 2004;113:846–55.
Khosla S, Westendorf JJ, Oursler MJ. Building bone to reverse osteoporosis and repair fractures. J Clin Investig. 2008;118:421–8.
Haberland M, Schilling AF, Rueger JM, Amling M. Brain and bone: central regulation of bone mass. A new paradigm in skeletal biology. J Bone Joint Surg Am. 2001;83-A:1871–6.
Egermann M, Goldhahn J, Schneider E. Animal models for fracture treatment in osteoporosis. Osteoporos Int. 2005;16(Suppl 2):S129–38.
Bonjour JP, Ammann P, Rizzoli R. Importance of preclinical studies in the development of drugs for treatment of osteoporosis: a review related to the 1998 WHO guidelines. Osteoporos Int. 1999;9:379–93.
Turner AS. Animal models of osteoporosis–necessity and limitations. Eur Cell Mater. 2001;1:66–81.
Lelovas PP, Xanthos TT, Thoma SE, Lyritis GP, Dontas IA. The laboratory rat as an animal model for osteoporosis research. Comp Med. 2008;58:424–30.
Boyd D, Carroll G, Towler MR, Freeman C, Farthing P, et al. Preliminary investigation of novel bone graft substitutes based on strontium-calcium-zinc-silicate glasses. J Mater Sci Mater Med. 2009;20:413–20.
Bossini PS, Renno AC, Ribeiro DA, Fangel R, Peitl O, et al. Biosilicate(R) and low-level laser therapy improve bone repair in osteoporotic rats. J Tissue Eng Regen Med. 2011;5:229–37.
Li Y, Li Q, Zhu S, Luo E, Li J, et al. The effect of strontium-substituted hydroxyapatite coating on implant fixation in ovariectomized rats. Biomaterials. 2010;31:9006–14.
Kumar A, Gupta GK, Khedgikar V, Gautam J, Kushwaha P, et al. In vivo efficacy studies of layer-by-layer nano-matrix bearing kaempferol for the conditions of osteoporosis: a study in ovariectomized rat model. Eur J Pharm Biopharm. 2012;82:508–17.
Zhang Y, Cheng N, Miron R, Shi B, Cheng X. Delivery of PDGF-B and BMP-7 by mesoporous bioglass/silk fibrin scaffolds for the repair of osteoporotic defects. Biomaterials. 2012;33:6698–708.
Ikeda T, Yamaguchi A, Yokose S, Nagai Y, Yamato H, et al. Changes in biological activity of bone cells in ovariectomized rats revealed by in situ hybridization. J Bone Miner Res. 1996;11:780–8.
Jiang JM, Sacco SM, Ward WE. Ovariectomy-induced hyperphagia does not modulate bone mineral density or bone strength in rats. J Nutr. 2008;138:2106–10.
Kaplan DL, Altman GH, Diaz F, Jakuba C, Calabro T, et al. Silk-based biomaterials. Biomaterials. 2003;24:401–16.
Barbieri D, Renard AJS, de Bruijn JD, Yuan H. Heterotopic bone formation by nano-apatite containing poly(d,l-Lactide) composites. Eur Cells Mater. 2010;19:252–61.
den Boer FC, Wippermann BW, Blokhuis TJ, Patka P, Bakker FC, et al. Healing of segmental bone defects with granular porous hydroxyapatite augmented with recombinant human osteogenic protein-1 or autologous bone marrow. J Orthop Res. 2003;21:521–8.
Yang H, Wang G, Li M, Lu S, Chen X, et al. The use of silk fibroin/hydroxyapatite composite co-cultured with rabbit bone-marrow stromal cells in the healing of a segmental bone defect. J Bone Joint Surg Br Vol. 2010;92B:320–5.
He YX, Zhang G, Pan XH, Liu Z, Zheng LZ, et al. Impaired bone healing pattern in mice with ovariectomy-induced osteoporosis: a drill-hole defect model. Bone. 2011;48:1388–400.
Ramprasath VR, Shanthi P, Sachdanandam P. Curative effect of Semecarpus anacardium Linn. nut milk extract against adjuvant arthritis—with special reference to bone metabolism. Chem Biol Interact. 2006;160:183–92.
Cowles EA, DeRome ME, Pastizzo G, Brailey LL, Gronowicz GA. Mineralization and the expression of matrix proteins during in vivo bone development. Calcif Tissue Int. 1998;62:74–82.
Denhardt DT, Noda M, O’Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Investig. 2001;107:1055–61.
van Blitterswijk CA, Habibovic P, Yuan HP, van der Valk CM, Meijer G, et al. 3D microenvironment as essential element for osteoinduction by biomaterials. Biomaterials. 2005;26:3565–75.
Yuan H, Yang Z, Li Y, Zhang X, De Bruijn JD, et al. Osteoinduction by calcium phosphate biomaterials. J Mater Sci Mater Med. 1998;9:723–6.
Yuan H, Zou P, Yang Z, Zhang X, De Bruijn JD, et al. Bone morphogenetic protein and ceramic-induced osteogenesis. J Mater Sci Mater Med. 1998;9:717–21.
Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater. 2007;6:997–1003.
Zhu XD, Fan HS, Xiao YM, Li DX, Zhang HJ, et al. Effect of surface structure on protein adsorption to biphasic calcium-phosphate ceramics in vitro and in vivo. Acta Biomater. 2009;5:1311–8.
Acknowledgments
Funding for this study was provided by research grants from the Natural Science Foundation of China (30700948) to Yufeng Zhang and (81170992) to Bin Shi, and the Fundamental Research Fund for the Central Universities (2012304274818) to Ning Cheng.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ning Cheng and Jing Dai contribute equally to the study.
Rights and permissions
About this article
Cite this article
Cheng, N., Dai, J., Cheng, X. et al. Porous CaP/silk composite scaffolds to repair femur defects in an osteoporotic model. J Mater Sci: Mater Med 24, 1963–1975 (2013). https://doi.org/10.1007/s10856-013-4945-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-013-4945-y