Abstract
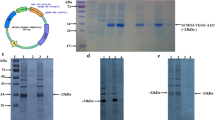
Tumstatin, a 28-kDa C-terminal fragment of collagen IV, is a potent anti-angiogenic protein and inhibitor of tumour growth. Recombinant tumstatin was prepared from Escherichia coli deposited as insoluble, inactive inclusion bodies. In the present study, we produced soluble and biologically active recombinant human tumstatin in E. coli by the coding region of tumstatin being linked to the 3′-end of the maltose-binding protein (MBP) gene. The fusion protein was expressed as the soluble form after induction by isopropylthio-β-D-galactoside (IPTG). MBP-tumstatin was purified by amylose affinity chromatography. MBP can be removed by digestion with factor Xa. Expression could represent 20% of the total soluble protein in E. coli, allowing approximately 8.6 mg of highly purified protein to be obtained per litre of bacterial culture. The purified tumstatin specifically inhibited the proliferation of endothelial cells in a dose-dependent manner. Annexin V-FITC apoptotic assay showed that recombinant tumstatin induced significant increase of apoptotic endothelial cells after 20 h of exposure to 20 μg/ml tumstatin, and when tumstatin was incubated on the chicken embryo, chorioallantoic membrane at doses of 1–15 μg, there was a dramatic decrease in microvasculaturethe allantoids of chicken embryos neovascular vessel test in vivo demonstrated that tumstatin treatment at doses of 1–15 μg gives rise to dramatically decrease the number of neovascular vessel. Our study provides a feasible and convenient approach to produce soluble and biologically active tumstatin.
Similar content being viewed by others
References
Folkman J (2001) In: Brauwald E (ed) Harrison’s textbook of internal medicine, 15th Edn, McGraw-Hill, New York, pp 517–530
Hanahan D, Folkman J (2000) The hallmarks of cancer. Cell 100:57–70
O’Reilly MS, Holmgren L, Shing Y et al (1994) Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 79:315–328
O’Reilly MS, Boehm T, Shing Y et al (1997) Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88:277–285
Kamphaus GD, Colorado PC, Panka DJ et al (2000) Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. J Biol Chem 275:1209–1215
Colorado PC, Torre A, Kamphaus G et al (2000) Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res 60:2520–2526
Maeshima Y, Colorado PC, Torre A et al (2000) Distinct antitumor properties of a type IV collagen domain derived from basement membrane. J Biol Chem 275:21340–21348
Luo YQ, Wang LH, Ma XL et al (2006) Construction, expression, and characterization of a new targeted bifunctional fusion protein: tumstatin45-132-TNF. IUBMB Life 58:647–653
Sambrook J, Fritsch EF, Maniatis T (2001) A laboratory manual. Cold Spring Harbor Press, New York, pp 66–87
van Engeland M, Nieland LJ, Ramaekers FC et al (1998) Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidyl serine exposure. Cytometry 1:1–9
Nguyen M, Shing Y, Folkman J (1994) Quantitation of angiogenesis and antiangiogenesis in the chick embryo chorioallantoic membrane. Microvasc Res 47:31–40
Maeshima Y, Sudhakar A, Lively JC et al (2002) Tumstatin, an endothelial cell-specific inhibitor of protein synthesis. Science 295:140–143
Folkman J (1995) Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1:27–31
Moser TL, Stack MS, Wahl ML et al (2002) The mechanism of action of angiostatin: can you teach an old dog new tricks? Thromb Haemost 87:394–401
Mukhopadhyay A (1997) Inclusion bodies and purification of proteins in biologically active forms. Adv Biochem Eng Biotechnol 56:61–109
Nagai K, Perutz MF, Poyart C (1985) Oxygen binding properties of human mutant hemoglobins synthesized in Escherichia coli. Proc Natl Acad Sci U S A 82:7252–7255
Quinlan RA, Moir RD, Stewart M (1989) Expression in Escherichia coli of fragments of glial fibrillary acidic protein: characterization, assembly properties and paracrystal formation. J Cell Sci 93:71–83
Eaton D, Rodriguez H, Vehar GA (1986) Proteolytic processing of human factor VIII. Correlation of specific cleavages by thrombin, factor Xa, and activated protein C with activation and inactivation of factor VIII coagulant activity. Biochemistry 25:505–512
Wearne SJ (1990) Factor Xa cleavage of fusion proteins. Elimination of non-specific cleavage by reversible acylation. FEBS Lett 263:23–26
Kapust RB, Waugh DS (1999) Escherichia coli maltose-binding protein is uncommonly effective at promoting the solubility of polypeptides to which it is fused. Protein Sci 8:1668–1674
Fox JD, Kapust RB, Waugh DS (2001) Single amino acid substitutions on the surface of Escherichia coli maltose-binding protein can have a profound impact on the solubility of fusion proteins. Protein Sci 10:622–630
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, YQ., Wang, LH., Yi, Q. et al. Expression of soluble, biologically active recombinant human tumstatin in Escherichia coli . Clin. Exper.Med. 8, 37–42 (2008). https://doi.org/10.1007/s10238-008-0154-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-008-0154-2