Abstract
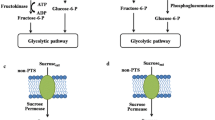
Sucrose is one of the most promising carbon sources for industrial fermentation. To achieve sucrose catabolism, the sucrose utilization operons have been introduced into microorganisms that are not able to utilize sucrose. However, the rates of growth and sucrose uptake of these engineered strains were relatively low to be successfully employed for industrial applications. Here, we report a practical example of developing sucrose-utilizing microorganisms using Escherichia coli K-12 as a model system. The sucrose utilizing ability was acquired by introducing only β-fructofuranosidase from three different sucrose-utilizing organisms (Mannheimia succiniciproducens, E. coli W, and Bacillus subtilis). Among them, the M. succiniciproducens β-fructofuranosidase was found to be the most effective for sucrose utilization. Analyses of the underlying mechanism revealed that sucrose was hydrolyzed into glucose and fructose in the extracellular space and both liberated hexoses could be transported by their respective uptake systems in E. coli K-12. To prove that this system can also be applied for the production of useful metabolites, the M. succiniciproducens β-fructofuranosidase was introduced into the engineered l-threonine production strain of E. coli K-12. This recombinant strain was able to produce 51.1 g/L l-threonine by fed-batch culture, resulting in an overall yield of 0.284 g l-threonine per g sucrose. This simple approach to make E. coli K-12 to acquire sucrose-utilizing ability and its successful biotechnological application can be employed to develop sustainable bioprocesses using renewable biomass.




Similar content being viewed by others
References
Antelmann H, Tjalsma H, Voigt B, Ohlmeier S, Bron S, van Dijl JM, Hecker M (2001) A proteomic view on genome–based signal peptide predictions. Genome Res 11:1484–1502
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Emanuelsson O, Brunak S, von Heijne G, Nielsen H (2007) Locating proteins in the cell using TargetP, SignalP and related tools. Nat Protoc 2:953–971
Garcia JL (1985) Cloning in Escherichia coli and molecular analysis of the sucrose system of the Salmonella plasmid SCR-53. Mol Gen Genet 201:575–577
Hirose I, Sano K, Shioda I, Kumano M, Nakamura K, Yamane K (2000) Proteome analysis of Bacillus subtilis extracellular proteins: a two-dimensional protein electrophoretic study. Microbiology 146:65–75
Hovatta O, Silye R, Krausz T, Abir R, Margara R, Trew G, Lass A, Winston RM (1996) Cryopreservation of human ovarian tissue using dimethylsulphoxide and propanediol–sucrose as cryoprotectants. Hum Reprod 11:1268–1272
Jahreis K, Bentler L, Bockmann J, Hans S, Meyer A, Siepelmeyer J, Lengeler JW (2002) Adaptation of sucrose metabolism in the Escherichia coli wild-type strain EC3132. J Bacteriol 184:5307–5316
Jeffery CJ (2003) Moonlighting proteins: old proteins learning new tricks. Trends Genet 19:415–417
Kilimann KV, Doster W, Vogel RF, Hartmann C, Ganzle MG (2006) Protection by sucrose against heat-induced lethal and sublethal injury of Lactococcus lactis: an FT-IR study. Biochim Biophys Acta 1764:1188–1197
Kim JM, Lee KH, Lee SY (2008) Development of a markerless gene knock-out system for Mannheimia succiniciproducens using a temperature–sensitive plasmid. FEMS Microbiol Lett 278:78–85
Koutinas AA, Wang R, Webb C (2004) Evaluation of wheat as generic feedstock for chemical production. Ind Crop Prod 20:75–88
Lee JW, Lee SY, Song H, Yoo JS (2006a) The proteome of Mannheimia succiniciproducens, a capnophilic rumen bacterium. Proteomics 6:3550–3566
Lee SJ, Song H, Lee SY (2006b) Genome-based metabolic engineering of Mannheimia succiniciproducens for succinic acid production. Appl Environ Microbiol 72:1939–1948
Lee KH, Park JH, Kim TY, Kim HU, Lee SY (2007) Systems metabolic engineering of Escherichia coli for l-threonine production. Mol Syst Biol 3:149
Li M, Rosenshine I, Tung SL, Wang XH, Friedberg D, Hew CL, Leung KY (2004) Comparative proteomic analysis of extracellular proteins of enterohemorrhagic and enteropathogenic Escherichia coli strains and their ihf and ler mutants. Appl Environ Microbiol 70:5274–5282
Molina-Hoppner A, Doster W, Vogel RF, Ganzle MG (2004) Protective effect of sucrose and sodium chloride for Lactococcus lactis during sublethal and lethal high-pressure treatments. Appl Environ Microbiol 70:2013–2020
Ould-Moulaye CB, Dussap CG, Gros JB (1999) Estimation of Gibbs energy changes of central metabolism reactions. Biotechnol Tech 13:187–193
Park JH, Lee KH, Kim TY, Lee SY (2007) Metabolic engineering of Escherichia coli for the production of L-valine based on transcriptome analysis and in silico gene knockout simulation. Proc Natl Acad Sci USA 104:7797–7802
Prestrelski SJ, Tedeschi N, Arakawa T, Carpenter JF (1993) Dehydration-induced conformational transitions in proteins and their inhibition by stabilizers. Biophys J 65:661–671
Qian ZG, Xia XX, Lee SY (2009) Metabolic engineering of Escherichia coli for the production of putrescine: a four carbon diamine. Biotechnol Bioeng 104:651–662
Reid SJ, Abratt VR (2005) Sucrose utilisation in bacteria: genetic organisation and regulation. Appl Microbiol Biotechnol 67:312–321
Renouf MA, Wegener MK, Nielsen LK (2008) An environmental life cycle assessment comparing Australian sugarcane with US corn and UK sugar beet as producers of sugars for fermentation. Biomass Bioenergy 32:1144–1155
Roy I, Gupta MN (2004) Freeze-drying of proteins: some emerging concerns. Biotechnol Appl Biochem 39:165–177
Sahin-Toth M, Lengyel Z, Tsunekawa H (1999) Cloning, sequencing, and expression of cscA invertase from Escherichia coli B-62. Can J Microbiol 45:418–422
Sambrook JRD (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Lab Press, Cold Spring Harbor
Schmid K, Schupfner M, Schmitt R (1982) Plasmid-mediated uptake and metabolism of sucrose by Escherichia coli K-12. J Bacteriol 151:68–76
Schmid K, Ebner R, Altenbuchner J, Schmitt R, Lengeler JW (1988) Plasmid-mediated sucrose metabolism in Escherichia coli K12: mapping of the scr genes of pUR400. Mol Microbiol 2:1–8
Scholle RR, Coyne VE, Maharaj R, Robb FT, Woods DR (1987) Expression and regulation of a Vibrio alginolyticus sucrose utilization system cloned in Escherichia coli. J Bacteriol 169:2685–2690
Scholle RR, Steffen HE, Goodman HJ, Woods DR (1990) Expression and regulation of a Bacteroides fragilis sucrose utilization system cloned in Escherichia coli. Appl Environ Microbiol 56:1944–1948
Sprenger GA, Lengeler JW (1988) Analysis of sucrose catabolism in Klebsiella pneumoniae and in Scr + derivatives of Escherichia coli K12. J Gen Microbiol 134:1635–1644
Tsunekawa H, Azuma S, Okabe M, Okamoto R, Aiba S (1992) Acquisition of a sucrose utilization system in Escherichia coli K-12 derivatives and its application to industry. Appl Environ Microbiol 58:2081–2088
Van Opstal I, Vanmuysen SC, Michiels CW (2003) High sucrose concentration protects E. coli against high pressure inactivation but not against high pressure sensitization to the lactoperoxidase system. Int J Food Microbiol 88:1–9
Vizcaino C, Restrepo-Montoya D, Rodriguez D, Nino LF, Ocampo M, Vanegas M, Reguero MT, Martinez NL, Patarroyo ME, Patarroyo MA (2010) Computational prediction and experimental assessment of secreted/surface proteins from mycobacterium tuberculosis H37Rv. PLoS Comput Biol 6:e1000824
Wohlhieter JA, Lazere JR, Snellings NJ, Johnson EM, Synenki RM, Baron LS (1975) Characterization of transmissible genetic elements from sucrose-fermenting Salmonella strains. J Bacteriol 122:401–406
Xia XX, Han MJ, Lee SY, Yoo JS (2008) Comparison of the extracellular proteomes of Escherichia coli B and K-12 strains during high cell density cultivation. Proteomics 8:2089–2103
Acknowledgements
This work was supported by the Korea–Australia Collaborative Research Project on the Development of Sucrose-Based Bioprocess Platform (10030795) from the Korean Ministry of Knowledge Economy. Further support by World Class University program (R322009000101420) by the Ministry of Education, Science and Technology through the National Research Foundation is appreciated.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lee, J.W., Choi, S., Park, J.H. et al. Development of sucrose-utilizing Escherichia coli K-12 strain by cloning β-fructofuranosidases and its application for l-threonine production. Appl Microbiol Biotechnol 88, 905–913 (2010). https://doi.org/10.1007/s00253-010-2825-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2825-7