Abstract
Teriparatide (TPTD), a recombinant human parathyroid hormone N-terminal fragment (1–34), is a widely used bone anabolic drug for osteoporosis. Sequential treatment with antiresorptives such as bisphosphonates after TPTD discontinuation is generally recommended. However, relative effects of bisphosphonates have not been determined. In the present study, we directly compared effects of risedronate (RIS) and alendronate (ALN) on bone mineral density (BMD), bone turnover, structural property and strength in ovariectomized (OVX) rats, when administered after TPTD. Female Sprague Dawley rats were divided into one sham-operated and eight ovariectomized groups. TPTD, RIS, and ALN were given subcutaneously twice per week for 4 or 8 weeks after 4 week treatment with TPTD. TPTD significantly increased BMD (+9.6%) in OVX rats after 4 weeks of treatment. 8 weeks after TPTD withdrawal, vehicle-treated group showed a blunted BMD increase of +8.4% from the baseline. In contrast, 8 weeks of treatment with RIS and ALN significantly increased BMD to 17.4 and 21.8%, respectively. While ALN caused a consistently larger increase in BMD, sequential treatment with RIS resulted in lower Tb.Sp compared to ALN in the fourth lumbar vertebra as well as in greater stiffness in compression test. In conclusion, the present study demonstrated that sequential therapy with ALN and RIS after TPTD both improved bone mass and structure. Our results further suggest that RIS may have a greater effect on improving bone quality and stiffness than ALN despite less prominent effect on BMD. Further studies are necessary to determine clinical relevance of these findings to fracture rate.
Similar content being viewed by others
Introduction
Teriparatide (TPTD), an N-terminal fragment of human parathyroid hormone (PTH) (1–34), is an anabolic agent for the treatment of osteoporosis [1–3] that markedly increases bone mineral density (BMD) and decreases fracture risk [4–6]. It has been shown to be even more potent particularly in lumbar BMD gains than standard antiresorptives such as alendronate (ALN) and raloxifene [7–10]. However, duration of TPTD therapy is restricted to 2 years during lifetime due to a concern about development of osteosarcoma [11, 12]. Moreover, discontinuation of TPTD treatment results in substantial loss of the BMD gains by the drug. It is thus recommended to sequentially use antiresorptives following TPTD therapy to maintain bone mass [4, 13, 14].
Several studies have investigated efficacy of concurrent or sequential treatment with TPTD and antiresorptives including bisphosphonates [15–23]. Contrary to an initial expectation that simultaneous treatment with anabolic and antiresorptive agents would result in a greater BMD increase in an additive or synergistic manner, a combination therapy of TPTD and ALN turned out to be less efficacious than TPTD monotherapy [16]. Sequential treatment with TPTD after antiresorptives also resulted in diminished effects. The blunted response to TPTD by concomitant or prior treatment with antiresorptives seemed in part due to the fact that the anabolic effect of TPTD depends on bone remodeling activity [23–26]. Exceptionally, however, zoledronate and denosumab showed additive effect when used in combination with TPTD [9, 27] despite their strong antiresorptive activities. Thus, the mechanism of bone anabolic effects of TPTD and its interactions with antiresorptive agents remain to be elucidated.
Risedronate (RIS) and ALN are nitrogen-containing bisphosphonates widely used as first-line drugs for the treatment of osteoporosis. Although these two bisphosphonates share the fundamental mechanism of inhibiting bone resorption [28], they significantly differ in potency of inhibiting farnesyl pyrophosphate synthase and affinity to hydroxyapatite [29]. Such unique properties may explain differences in clinical efficacy of the two bisphosphonates; despite its less prominent effect on bone turnover and BMD than ALN, RIS appears similarly to or even more effective in preventing hip and non-vertebral fractures during the first year of therapy [30] and in experimental animals as well under certain conditions [31]. As for sequential therapy, 1-year TPTD therapy after prior treatment with RIS or ALN for at least 2 years significantly increased BMD and bone formation markers, and the effect was greater in patients previously treated with RIS than in those pretreated with ALN [22]. It is also of note that a combination therapy with RIS and TPTD caused a greater effect than monotherapy in humans [32, 33]. In rodents, sequential treatment with RIS was able to maintain BMD gains by PTH pretreatment in ovariectomized (OVX) animals [34]. Similar results were obtained with ALN when administered after TPTD in rats [35]. It is currently unknown whether effects of sequential treatment with bisphosphonates after TPTD therapy differ among different bisphosphonates.
Thus, in the present study, effects of sequential treatment with RIS and ALN after TPTD therapy were directly compared in OVX rats.
Materials and Methods
Experimental Animals
Seventy two female Sprague–Dawley (SD) rats at 7 weeks of age were used (Beijing Vital River, Beijing, China). The animals were housed two per cage in a room with light/dark cycle of 12/12 h and were acclimated to study condition for 5 weeks prior to the start of the experiment. All animals were pair-fed rodent food (irradiated, Shanghai SLAC Laboratory Animal Co. Ltd., Shanghai, China). Water from PharmaLegacy Laboratories Co. Ltd. (Shanghai, China) in house production was available to animals ad libitum. The experimental protocols were approved by Animal Care and Use Committee of EA Pharma Co. Ltd. and PharmaLegacy Laboratories IACUC.
Materials
Risedronate (cat no. R521500) was purchased from Toronto Research Chemical (Toronto, ON, Canada). Alendronate (cat no. 126855) and teriparatide (cat no. 05235501) were purchased from EMD Biosciences (Darmstadt, Germany). Atropine (cat no. A0132), calcein (cat no. C0875), buprenorphine (cat no. B9275), gentamicin (cat no. G1914), and ketamine (cat no. K2753) were purchased from Sigma (St. Louis, MO, USA). Isoflurane (cat no. 1001936040) was purchased from Baxter (Deerfield, IL, USA).
Experimental Design
The experimental timeline is summarized in Fig. 1. The animals were assigned to sham-operated or ovariectomized (OVX) group by matching body weight and bone mineral density (BMD) of lumbar vertebrae (L2-L5) by BioBook system (IDBS, ID Business Solutions LTD., Shanghai, China). The OVX group was assigned to (1) vehicle, (2) TPTD, (3) TPTD and vehicle, (4) TPTD and RIS, (5) TPTD and ALN, (6) TPTD and vehicle, (7) TPTD and RIS, and (8) TPTD and ALN with eight animals in each group. The groups (1) and (2) were sacrificed at 4 weeks; (3), (4), and (5) were at 8 weeks; and (6), (7), (8), and the sham groups were at 12 weeks. TPTD (0.1 mg/kg, s.c.) was injected twice a week for 4 weeks. RIS (0.01 mg/kg, s.c.) or ALN (0.02 mg/kg, s.c.) was injected twice a week for 4 or 8 weeks after TPTD (Fig. 1).
Study timeline. Treatment was started from the next day after surgery. Animals were treated with vehicle or teriparatide (TPTD) twice a week for 4 weeks. After TPTD withdrawal, vehicle, risedronate (RIS), or alendronate (ALN) were given subcutaneously for 4 or 8 weeks. BMD, bone architecture, and bone strength was measured at 4, 8, or 12 weeks after surgery
Atropine (0.05 mg/kg, s.c.) was injected before surgery. Under anesthesia with ketamine (70 mg/kg, i.p.) and isoflurane 1–5% inhalation with a 0.8–1.5 L flow rate of oxygen, the animals were removed of the bilateral ovaries or sham-operated from a low abdominal approach. Animals received analgesics including but not limited to buprenorphine (0.05 mg/kg, i.m.) and gentamycin (20 mg/kg, i.m.) after surgery.
Four, 8, and 12 weeks after dosing, the animals were evaluated for BMD of lumbar vertebrae (L2-L5) by in vivo Dual-Energy X-ray Absorptiometry (DEXA, Hologic Discovery SL; Hologic, Inc., Waltham, MA, USA). After sacrifice at the end of each treatment protocol, BMD of the fourth lumbar vertebra was measured ex vivo by peripheral Dual-Energy X-ray Absorptiometry (pDEXA, Norland/Stratec, Pforzheim, Germany). They were also subjected to mechanical testing (L5) and histomorphometric analysis (L4).
Labeling and Histomorphometry
Fluorochrome dye of calcein was administered to quantify bone formation rates. Calcein (10 mg/kg, s.c.) was administered at 9 and 2 days prior to sacrifice. The L4 were collected and prepared by undecalcifying methods. The specimens were dehydrated through gradient ethanol and embedded in methyl methacrylate. The blocks were sectioned at about 5 μm in thickness for staining and histomorphometry. Static and dynamic histomorphometric analyses of the L4 were performed. One section was stained with Goldner’s trichrome for static parameter measurements, and another was kept unstained for dynamic parameter measurements. The histomorphometry was done using OsteoMeasure™ (OsteoMetrics, Inc., Decatur, GA) software program on a computer in conjunction with a Nikon E501 bright field and fluorescent microscope (Nikon Corporation, Tokyo, Japan) to measure the primary parameters: bone surface (BS, µm), osteoclast surface (Oc.S, μm), osteoclast number (N.Oc), osteoid surface (OS, μm), osteoblast surface (Ob.S, μm), and mineralizing surface (MS, μm). The following parameters were calculated from primary parameters: osteoid surface (OS/BS, %), number of osteoclasts per trabecular surface (N.Oc/BS, /mm), surface area of osteoclasts contacting the trabecular surface (Oc.S/BS, %), osteoblast surface (Ob.S/BS, %), mineralized surface (MS/BS, %), bone formation rate (BFR/BS, %/year), and mineral apposition rate (MAR, μm/day). Osteoclasts were identified as cells forming resorption lacunae at the bone surface. Structural indices were measured by these systems: bone volume (BV/TV, %), trabecular thickness (Tb.Th, μm), trabecular number (Tb.N, /mm), and trabecular separation (Tb.Sp, μm) [36].
Mechanical Testing
The fifth lumbar vertebrae (L5) and femurs were tested for bone strength by compression and three-point bending tests, respectively, using previously described methods with a slight modification [37]. The biomechanical testing was done using MTS858 (MTS System, Minneapolis, MN). For compression tests, the vertebra was fixed on a holder with adhesive agent and placed on the testing platform. For three-point bending of femur, the span of the two support points below the bone was 14 mm. The deformation rate was 6 mm/min for both femurs and vertebrae.
Load-deformation curves were transported to a personal computer and acquired by Team 490 software (version 4.10, Nicolet Instrument Technologies, Madison, WI). Sigma Plot 7.0 software (SPSS, Chicago, IL) was used to smooth the load-deformation curve and calculate the extrinsic material properties of the bone samples, including the maximal load (N) and linear stiffness (N/mm).
Statistical Analysis
All data were expressed as mean ± standard deviation (SD). According to the results of Shapiro–Wilk test for normal distribution, group differences were analyzed by t-test or Wilcoxon test and analysis of variance (ANOVA) with post hoc Dunnett’s test or Steel test on JMP (ver. 12.0.1, SAS Institute, Tokyo, Japan), and two-way ANOVA on Prism (ver. 6.07, Graph Pad Software, .CA, USA). P values less than 0.05 were considered statistically significant.
Results
In Vivo BMD of L2-L5 by DEXA
As shown in Fig. 2, the sham-operated rats gradually gained BMD throughout the study: 13.2, 15.8, and 18.6% at 4, 8, and 12 weeks, respectively. The vehicle-treated OVX rats only showed a 2.4% increase in BMD during the initial 4 weeks. In contrast, the TPTD-treated rats showed a significantly greater increase in BMD at 4 weeks (9.6%, p < 0.0002, compared with vehicle). After discontinuation of TPTD, the subsequent treatment with vehicle caused a gradual decline in BMD during 4–12 weeks. In sharp contrast, the rats sequentially treated with ALN or RIS following TPTD therapy attained the BMD level similar to the sham-operated animals and significantly higher than the TPTD vehicle-treated OVX rats at 12 weeks. At 8 weeks, only the TPTD-ALN group, showed a significantly higher BMD than that of TPTD-vehicle rats, suggesting that effect of ALN on BMD might be somewhat superior to that of RIS at the early phase of sequential treatment after TPTD.
Effect of sequential treatment with bisphosphonates after teriparatide on lumber vertebral BMD (L2-L5). Sham-operated rats are shown in open circles. Ovariectomized rats treated with vehicle for 4 weeks are shown in open squares. The other rats were first treated with teriparatide for 4 weeks, and then with vehicle (closed circles), risedronate (closed triangles), or alendronate (closed rhombuses) for up to 8 weeks. Percent increases from the baseline in lumber vertebral BMD (L2-L5), measured by in vivo DEXA are shown in mean ± SD. At 4 weeks, sham and vehicle: n = 8 and TPTD: n = 56. At 8 weeks, sham: n = 8 and TPTD→vehicle, TPTD→RIS, and TPTD→ALN: n = 16. At 12 weeks, sham, TPTD→vehicle, TPTD→RIS, and TPTD→ALN: n = 8. *** p < 0.001 and **** p < 0.0001 compared with the vehicle-treated group at the same time point
Ex Vivo BMD of L4 and Tibia by pDEXA
As shown in Fig. 3, ex vivo BMD scans by pDEXA gave similar results to those obtained by in vivo DEXA. During the first 4 weeks, TPTD significantly increased BMD compared with vehicle. After TPTD discontinuation, sequential treatment with either ALN or RIS resulted in a greater increase in L4 BMD compared with vehicle at 12 weeks. However, at 8 weeks, significant difference from the vehicle was only observed with ALN. And BMD at 12 weeks was significantly higher in TPTD-ALN rats than that in the TPTD-RIS group (Fig. 3). Thus, at least effect on lumbar BMD appeared greater with ALN than RIS when used in sequential therapy after TPTD.
Effect of sequential treatment with bisphosphonates after teriparatide on lumbar vertebral BMD (L4). After ovariectomy, rats were treated with vehicle or teriparatide for 4 weeks. After teriparatide withdrawal, risedronate or alendronate was given for another 4 or 8 weeks. Lumber vertebrae were harvested from the rats at 4, 8, or 12 weeks for ex vivo BMD measurements by pDEXA. Values of lumbar vertebral BMD (L4) are shown in mean ± SD. n = 8. **p < 0.01 and ****p < 0.0001 compared with the vehicle-treated group at same time point. c p < 0.001 significant difference between risedronate and alendronate
We also analyzed right tibia BMD and obtained virtually the same results: both ALN and RIS caused significantly greater increases than vehicle at 12 weeks, but ALN showed a slightly greater effect (data not shown).
Mechanical Testing
Vertebral compression tests revealed that treatment with TPTD for 4 weeks caused no significant changes in stiffness (Fig. 4a). And sequential therapy with RIS did not significantly affect vertebral stiffness at 8 and 12 weeks, either. Interestingly, although ALN-treated rats showed higher BMD at 12 weeks, stiffness was significantly lower compared with RIS (Fig. 4a). As for maximum load, initial treatment with TPTD and a subsequent treatment with RIS and ALN resulted in no appreciable changes compared to vehicle at each time point (Fig. 4b). Three-point bending tests revealed no statistically significant effects of these drugs on femoral strength (Table 1).
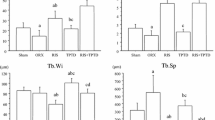
Effect of sequential treatment with bisphosphonates after teriparatide on a stiffness and b maximum load of lumber vertebra (L5). After ovariectomy, rats were treated with vehicle or teriparatide for 4 weeks. After teriparatide withdrawal, risedronate or alendronate was given for another 4 or 8 weeks. Lumber vertebrae (L5) were harvested from the rats at 4, 8, or 12 weeks and subjected to compression tests. Data are mean ± SD. n = 8. * p < 0.05 significant difference between risedronate and alendronate
Histomorphometry of Lumbar Vertebrae
As shown in Table 2, histomorphometric analysis of lumbar vertebrae revealed that Ob.S/BS, OS/BS, BFR/BS, and MS/BS were significantly increased by TPTD compared with vehicle at 4 weeks, confirming enhanced bone formation. And at 8 and 12 weeks, discontinuation of TPTD caused a significant decrease in these parameters.
Both RIS and ALN, administered after TPTD, significantly reduced parameters of bone turnover such as N.OC/BS, Ob.S/BS, and BFR/BS. As a result, these bisphosphonates improved bone volume and structural indices at 8 and 12 weeks, and a significant decrease was observed in Tb.Sp. Interestingly, RIS showed a significantly lower Tb.Sp compared with ALN at 12 weeks (Table 2). We also obtained similar results in the histomorphometric analysis of proximal tibia. Both bisphosphonates significantly suppressed turnover indices such as BFR/BS and increased BV/TV and structural parameters (data not shown). Tb.Sp at 12 weeks was lower in RIS compared with ALN: 569 ± 257, 209 ± 42, and 235 ± 58 μm in vehicle, RIS, and ALN, respectively. Thus, superior effect of RIS on trabecular quality appeared reproducible in two different types of bones.
Discussion
Duration of TPTD treatment is limited to 2 years in clinical practice [12]. And TPTD effect seems at least in part reversible because discontinuation of TPTD results in significant bone loss. Thus, it is important and generally recommended for clinicians to sequentially treat patients with antiresorptives after TPTD therapy. There is, however, little evidence available for selection of specific post-TPTD therapy. Given the lack of such evidence, particularly, for bisphosphonates, we directly compared effects of two most commonly prescribed oral bisphosphonates, RIS and ALN, as a post-TPTD sequential therapy in OVX rats. The results demonstrated that both bisphosphonates were effective in terms of bone turnover suppression, BMD increase, and improvement of structural properties. As for the difference between the two bisphosphonates, ALN seemed stronger in turnover suppression and BMD increase, whereas RIS exhibited partially greater improvement in microstructure and strength.
In the current experiments, we adopted the same ratio of RIS and ALN doses as that clinically used (1:2). Although we do not have sufficient evidence or rationale for such a protocol in rodents, we were indeed able to observe some differences between RIS and ALN under such an experimental condition. First, BMD measurements by either in vivo DEXA or ex vivo pDEXA revealed greater effect of ALN than RIS in increasing BMD after TPTD therapy. This is consistent with the fact that in regular clinical doses, ALN showed a greater efficacy than RIS both in terms of turnover suppression and BMD gains in human subjects [38].
Contrary to a simple assumption that such BMD gains result in improved bone strength, the more robust increment in BMD by ALN did not translate into better mechanical properties compared with RIS. In fact, 8 weeks of treatment with RIS after 4-week TPTD treatment resulted in better stiffness in the compression test despite the lower BMD at the same time point, compared to TPTD-ALN sequential treatment. We are well aware that the results may not be definitively conclusive considering significant variations in such mechanical tests. Importantly, however, we were also able to demonstrate significantly lower trabecular separation at the histological level in RIS-treated rats. And the analyses of the bones in two distinct sites, vertebrae and proximal tibiae, showed consistent results. Thus, we believe that the results are suggestive of preferential effect of RIS on structural properties independent of BMD.
Although the mechanism is unknown, potentially superior effect of RIS on bone quality has already been recognized: clinical data have suggested that RIS appears more effective than ALN in preventing hip and non-vertebral fractures during the first year of therapy. Although no direct comparison has been made prospectively, RIS-treated subjects have a lower incidence of hip fractures than those treated with ALN for 12 months (0.37 vs. 0.58%) [30]. RIS is known to have less affinity to hydroxyapatite and more potency in inhibiting farnesyl pyrophosphate synthase than ALN. Such differences have been hypothesized to cause distinct distribution pattern and local effects. For example, a previous study demonstrated deeper penetration of RIS into the bone tissue compared to ALN [39]. And we have indeed demonstrated actual differences in the effects of RIS and ALN on micro architecture in calcium-deficient OVX rats [31]. Thus, in the context of post-TPTD therapy, it seems plausible that RIS exhibited a distinct effect from ALN on bone microstructure, most likely with complex interaction with residual effect of prior TPTD treatment.
There are limitations in this study. First, morphological analysis was only done with classical histomorphometry [40], as we were unable to analyze the bone with micro CT that has widely been used in recent years. Another limitation is that we used younger rats at 12 weeks of age compared to previous studies [34]. Our results should be interpreted with caution and may not be applied to older animals.
Conclusions
In conclusion, we demonstrated that both RIS and ALN suppressed bone turnover, increased BMD, and improved structural properties in OVX rats when used for sequential treatment after TPTD. And we also found for the first time by direct comparison that ALN caused a stronger suppression of bone turnover and a more robust increase in BMD, whereas RIS treatment resulted in potentially better trabecular architecture and stiffness, suggesting preferential effect of RIS on bone quality. Whether such differences are indeed clinically relevant awaits fracture prevention studies by sequential treatment with different bisphosphonates after TPTD.
References
Hodsman AB, Bauer DC, Dempster DW, Dian L, Hanley DA, Harris ST, Kendler DL, McClung MR, Miller PD, Olszynski WP, Orwoll E, Yuen CK (2005) Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev 26:688–703
Trivedi R, Mithal A, Chattopadhyay N (2010) Anabolics in osteoporosis: the emerging therapeutic tool. Curr Mol Med 10:14–28
Cusano NE, Costa AG, Silva BC, Bilezikian JP (2011) Therapy of osteoporosis in men with teriparatide. J Osteoporos 2011:463675
Kaufman JM, Orwoll E, Goemaere S, San Martin J, Hossain A, Dalsky GP, Lindsay R, Mitlak BH (2005) Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int 16:510–516
Oswald AJ, Berg J, Milne G, Ralston SH (2014) Teriparatide treatment of severe osteoporosis reduces the risk of vertebral fracture compared with standard care in routine clinical practice. Calcif Tissue Int 94:176–182
Walsh JB, Lems WF, Karras D, Langdahl BL, Ljunggren O, Fahrleitner-Pammer A, Barrett A, Rajzbaum G, Jakob F, Marin F (2012) Effectiveness of teriparatide in women over 75 years of age with severe osteoporosis: 36-month results from the European Forsteo Observational study (EFOS). Calcif Tissue Int 90:373–383
Finkelstein JS, Leder BZ, Burnett SAM, Wyland JJ, Lee H, Paz AV, Gibson K, Neer RM (2006) Effects of teriparatide, alendronate, or both on bone turnover in osteoporotic men. J Clin Endocrinol Metab 91:2882–2887
Bouxsein ML, Chen P, Glass EV, Kallmes DF, Delmas PD, Mitlak BH (2009) Teriparatide and raloxifene reduce the risk of new adjacent vertebral fractures in postmenopausal women with osteoporosis. Results from two randomized controlled trials. J Bone Joint Surg Am 91:1329–1338
Cosman F, Eriksen EF, Recknor C, Miller PD, Guañabens N, Kasperk C, Papanastasiou P, Readie A, Rao H, Gasser JA, Bucci-Rechtweg C, Boonen S (2011) Effects of intravenous zoledronic acid plus subcutaneous teriparatide [rhPTH(1–34)] in postmenopausal osteoporosis. J Bone Miner Res 26:503–511
Shen L, Xie X, Su Y, Luo C, Zhang C, Zeng B (2011) Parathyroid hormone versus bisphosphonate treatment on bone mineral density in osteoporosis therapy: a meta-analysis of randomized controlled trials. PloS ONE 6:e26267
Vahle JL, Sato M, Long GG, Young JK, Francis PC, Engelhardt JA, Westmore MS, Linda Y, Nold JB (2002) Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1–34) for 2 years and relevance to human safety. Toxicol Pathol 30:312–321
Tashjian AH Jr, Chabner BA (2002) Commentary on clinical safety of recombinant human parathyroid hormone 1–34 in the treatment of osteoporosis in men and postmenopausal women. J Bone Miner Res 17:1151–1161
Adami S, San Martin J, Muñoz-Torres M, Econs MJ, Xie L, Dalsky GP, McClung M, Felsenberg D, Brown JP, Brandi ML, Sipos A (2008) Effect of raloxifene after recombinant teriparatide [hPTH(1–34)] treatment in postmenopausal women with osteoporosis. Osteoporos Int 19:87–94
Andreopoulou P, Bockman RS (2015) Management of postmenopausal osteoporosis. Annu Rev Med 66:329–342
Cosman F, Nieves J, Woelfert L, Shen V, Lindsay R (1998) Alendronate does not block the anabolic effect of PTH in postmenopausal osteoporotic women. J Bone Miner Res 13:1051–1055
Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, Garnero P, Bouxsein ML, Bilezikian JP, Rosen CJ, PaTH Study Investigators (2003) The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 349:1207–1215
Black DM, Bilezikian JP, Ensrud KE, Greenspan SL, Palermo L, Hue T, Lang TF, McGowan JA, Rosen CJ, PaTH Study Investigators (2005) One year of alendronate after one year of parathyroid hormone (1–84) for osteoporosis. N Engl J Med 353:555–565
Vestergaard P, Jorgensen NR, Mosekilde L, Schwarz P (2007) Effects of parathyroid hormone alone or in combination with antiresorptive therapy on bone mineral density and fracture risk - a meta-analysis. Osteoporos Int 18:45–57
Antoniucci DM, Sellmeyer DE, Bilezikian JP, Palermo L, Ensrud KE, Greenspan SL, Black DM (2007) Elevations in serum and urinary calcium with parathyroid hormone (1–84) with and without alendronate for osteoporosis. J Clin Endorcinol Metab 92:942–947
Rittmaster RS, Bolognese M, Ettinger MP, Hanley DA, Hodsman AB, Kendler DL, Rosen CJ (2000) Enhancement of bone mass in osteoporotic women with parathyroid hormone followed by alendronate. J Clin Endocrinol Metab 85:2129–2134
Ettinger B, San Martin J, Crans G, Pavo I (2004) Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res 19:745–751
Miller PD, Delmas PD, Lindsay R, Watts NB, Luckey M, Adachi J, Saag K, Greenspan SL, Seeman E, Boonen S, Meeves S, Lang TF, Bilezikian JP Open-label study to determine how prior therapy with Alendronate or Risedronate in postmenopausal women with osteoporosis influences the clinical effectiveness of teriparatide investigators (2008) Early responsiveness of women with osteoporosis to teriparatide after therapy with alendronate or risedronate. J Clin Endocrinol Metab 93: 3785–3793.
Cosman F (2014) Anabolic and antiresorptive therapy for osteoporosis: combination and sequential approaches. Curr Osteoporos Rep 12:385–395
Jakob F, Oertel H, Langdahl B, Ljunggren O, Barrett A, Karras D, Walsh JB, Fahrleitner-Pammer A, Rajzbaum G, Barker C, Lems WF, Marin F (2012) Effects of teriparatide in postmenopausal women with osteoporosis pre-treated with bisphosphonates: 36-month results from the European forsteo observational study. Eur J Endocrinol 166:87–97
Eastell R, Nickelsen T, Marin F, Barker C, Hadji P, Farrerons J, Audran M, Boonen S, Brixen K, Gomes JM, Obermayer-Pietsch B, Avramidis A, Sigurdsson G, Glüer CC (2009) Sequential treatment of severe postmenopausal osteoporosis after teriparatide: final results of the randomized, controlled European study of forsteo (EUROFORS). J Bone Miner Res 24:726–736
Obermayer-Pietsch BM, Marin F, McCloskey EV, Hadji P, Farrerons J, Boonen S, Audran M, Barker C, Anastasilakis AD, Fraser WD, Nickelsen T, EUROFORS Investigators (2008) Effects of two years of daily teriparatide treatment on BMD in postmenopausal women with severe osteoporosis with and without prior antiresorptive treatment. J Bone Miner Res 23: 1591–1600
Tsai JN, Uihlein AV, Burnett-Bowie SM, Neer RM, Derrico NP, Lee H, Bouxsein ML, Leder BZ (2016) Effects of two years of teriparatide, denosumab, or both on bone microarchitecture and strength (DATA-HRpQCT study). J Clin Endocrinol Metab 101:2023–2030
Ebetino FH, Hogan AM, Sun S, Tsoumpra MK, Duan X, Triffitt JT, Kwaasi AA, Dunford JE, Barnett BL, Oppermann U, Lundy MW, Boyde A, Kashemirov BA, McKenna CE, Russell RG (2011) The relationship between the chemistry and biological activity of the bisphosphonates. Bone 49:20–33
Nancollas GH, Tang R, Phipps RJ, Henneman Z, Gulde S, Wu W, Mangood A, Russell RG, Ebetino FH (2006) Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone 38:617–627
Silverman SL, Watts NB, Delmas PD, Lange JL, Lindsay R (2007) Effectiveness of bisphosphonates on nonvertebral and hip fractures in the first year of therapy: the risedronate and alendronate (REAL) cohort study. Osteoporos Int 18:25–34
Yano T, Yamada M, Konda T, Shiozaki M, Inoue D (2014) Risedronate improves bone architecture and strength faster than alendronate in ovariectomized rats on a low-calcium diet. J Bone Miner Metab 32:653–659
Walker MD, Cusano NE, Sliney J Jr, Romano M, Zhang C, McMahon DJ, Bilezikian JP (2013) Combination therapy with risedronate and teriparatide in male osteoporosis. Endocr 44:237–246
Chen LX, Zhou ZR, Li YL, Ning GZ, Zhang TS, Zhang D, Feng SQ (2015) Comparison of bone mineral density in lumbar spine and fracture rate among eight drugs in treatments of osteoporosis in men: a network meta-analysis. PloS ONE 10:e0128032
Samnegård E, Iwaniec UT, Cullen DM, Kimmel DB, Recker RR (2001) Maintenance of cortical bone in human parathyroid hormone (1–84)-treated ovariectomized rats. Bone 28:251–260
Amugongo SK, Yao W, Jia J, Dai W, Lay YA, Jiang L, Harvey D, Zimmermann EA, Schaible E, Dave N, Ritchie RO, Kimmel DB, Lane NE (2014) Effect of sequential treatments with alendronate, parathyroid hormone (1–34) and raloxifene on cortical bone mass and strength in ovariectomized rats. Bone 67:257–268
Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 28:2–17
Ohnishi H, Nakamura T, Narusawa K, Murakami H, Abe M, Barbier A, Suzuki K (1997) Bisphosphonate tiludronate increases bone strength by improving mass and structure in established osteopenia after ovariectomy in rats. Bone 21:335–343
Reid DM, Hosking D, Kendler D, Brandi ML, Wark JD, Marques-Neto JF, Weryha G, Verbruggen N, Hustad CM, Mahlis EM, Melton ME (2008) A comparison of the effect of alendronate and risedronate on bone mineral density in postmenopausal women with osteoporosis: 24-month results from FACTS-International. Int J Clin Pract 62:575–584
Roelofs AJ, Stewart CA, Sun S, Błażewska KM, Kashemirov BA, McKenna CE, Russell RG, Rogers MJ, Lundy MW, Ebetino FH, Coxon FP (2012) Influence of bone affinity on the skeletal distribution of fluorescently labeled bisphosphonates in vivo. J Bone Miner Res 27:835–847
Li M, Mosekilde L, Søgaard CH, Thomsen JS, Wronski TJ (1995) Parathyroid hormone monotherapy and cotherapy with antiresorptive agents restore vertebral bone mass and strength in aged ovariectomized rats. Bone 16:629–635
Acknowledgements
We thank Mei-Shu Shih and Mei Yu, who were working for PharmaLegacy Laboratories, for helping us with animal experiments.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors Tetsuo Yano and Mei Yamada are employees of EA Pharma Co., Ltd. and Ajinomoto Co., Inc, respectively. Daisuke Inoue served as a consultant for EA Pharma.
Human and animal rights and informed consent
This article does not contains any studies with human participants performed by any of the authors. All procedures performed in the study were approved by the Animal Care and Use Committee of EA Pharma Co. Ltd. and PharmaLegacy Laboratories IACUC.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Yano, T., Yamada, M. & Inoue, D. Effect of Sequential Treatment with Bisphosphonates After Teriparatide in Ovariectomized Rats: A Direct Comparison Between Risedronate and Alendronate. Calcif Tissue Int 101, 102–110 (2017). https://doi.org/10.1007/s00223-017-0263-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-017-0263-6