Abstract
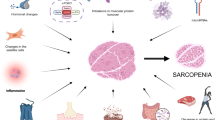
Skeletal muscle weakness is a leading cause of mobility disability in the elderly (sarcopenia), as a complication of acute or chronic illness (cachexia), and due to inherited or acquired muscle diseases (muscular dystrophies, myositides, etc.). As of now, there are no approved drugs that can reliably increase muscle strength and function. However, with our understanding of the regulation of myocyte signaling and homeostasis evolving rapidly, experimental treatments are now entering the clinic. We review the current status of clinical research in pharmacological therapies for muscle disorders.


Similar content being viewed by others
References
Pedersen BK (2011) Muscles and their myokines. J Exp Biol 214:337–346
Wenz T, Rossi SG, Rotundo RL, Speigelman BM, Moraes CT (2009) Increased muscle PGC-1α expression protects from sarcopenia and metabolic disease during aging. Proc Nat Acad Sci USA 106:20405–20410
Kelley DE, Goodpaster B, Wing RR, Simoneau JA (1999) Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol 277:E1130–E1141
Mitch WE, Goldberg AL (1996) Mechanisms of muscle wasting: the role of the ubiquitin-proteasome pathway. N Engl J Med 335:1897–1905
Egerman MA, Glass DJ (2014) Signaling pathways controlling skeletal muscle mass. Crit Rev Biochem Mol Biol 49:59–68
Markert CD, Ambrosio F, Call JA, Grange RW (2011) Exercise and Duchenne muscular dystrophy: toward evidence-based exercise prescription. Muscle Nerve 43:464–478
Emery AEH (2002) The muscular dystrophies. Lancet 359:687–695
Flanigan KM (2012) The muscular dystrophies. Semin Neurol 32:255–263
Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL (1993) Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA 90:3710–3714
Lynch GS, Rafael JA, Chamberlain JS, Faulkner JA (2000) Contraction-induced injury to single permeabilized muscle fibers from mdx, transgenic mdx, and control mice. Am J Physiol Cell Physiol 279:C1290–C1294
Turner PR, Westwood T, Regen CM, Steinhardt RA (1988) Increased protein degradation results from elevated free calcium levels found in muscle from mdx mice. Nature 335:735–738
Wallace GQ, McNally EM (2009) Mechanisms of muscle degeneration, regeneration, and repair in muscular dystrophies. Annu Rev Physiol 71:37–57
Ferlini A, Neri M, Gualandi F (2013) The medical genetics of dystrophinopathies: molecular genetic diagnosis and its impact on clinical practice. Neuromusc Disord 23:4–14
Koenig M, Beggs AH, Moyer M et al (1989) The molecular basis for Duchenne versus Becker muscular dystrophy: correlation of severity with type of deletion. Am J Hum Genet 45:498–506
Wagner KR, Mcpherron AC, Winik N, Lee SJ (2002) Loss of myostatin attenuates severity of muscular dystrophy in mdx mice. Ann Neurol 52:832–836
Morine KJ, Bish LT, Pendrak K, Sleeper MM, Barton ER, Sweeney HL (2010) Systemic myostatin inhibition via liver-targeted gene transfer in normal and dystrophic mice. PLoSOne 5:e9176
Morine KJ, Bish LT, Selsby JT, Gazzara JA, Pendrak K, Sleeper MM, Barton ER, Lee SJ, Sweeney HL (2010) Activin IIB receptor blockade attenuates dystrophic pathology in a mouse model of Duchenne muscular dystrophy. Muscle Nerve 42:722–730
Pistilli EE, Bogdanovich S, Goncalves MD, Ahima RS, Lachey J, Seehra J, Khurana T (2011) Targeting the activin type IIB receptor to improve muscle mass and function in the mdx mouse model of Duchenne muscular dystrophy. Am J Pathol 178:1287–1297
Nakatani M, Takehara Y, Sugino H, Hashimoto O, Hasegawa Y, Murakami T, Uezumi A, Takeda S, Noji S, Sunada Y, Tsuchida K (2008) Transgenic expression of a myostatin inhibitor derived from follistatin increases skeletal muscle mass and ameliorates dystrophic pathology in mdx mice. FASEB J 22:477–487
Bernasconi P, Di Blasi C, Mora M, Morandi L, Galbiati S, Confalonieri P, Cornelio F, Mantegazza R (1999) Transforming growth factor-β1 and fibrosis in congenital muscular dystrophies. Neuromuscul Disord 9:28–33
MacDonald EM, Cohn RD (2012) TGFβ signaling: its role in fibrosis formation and myopathies. Curr Opin Rheumatol 24:628–634
Nelson CA, Hunter RB, Quigley LA, Girgenrath S, Weber WD, McCullough JA, Dinardo CJ, Keefe KA, Ceci L, Clayton NP, McVie-Wylie A, Cheng SH, Leonard JP, Wentworth BM (2011) Inhibiting TGF-β activity improves respiratory function in mdx mice. Am J Pathol 178:2611–2621
Miyazono K, Olofsson A, Colosetti P, Heldin CH (1991) A role of the latent TGF-beta 1-binding protein in the assembly and secretion of TGF-beat 1. EMBO J 10:1091–1101
Heydemann A, Ceco E, Lim JE, Hadhazy M, Ryder P, Moran JL, Beier DR, Palmer AA, McNally E (2009) Latent TGF-β–binding protein 4 modifies muscular dystrophy in mice. J Clin Invest. 119:3703–3712
Flanigan KM, Ceco E, Lamar KM, Kaminoh I, Dunn DM, Mendell JR, King WM, Pestronk A, Florence JM, Florence JM, Mathews KD, Finkel RS, Swoboda KJ, Gappmaier E, Howard MT, Day JW, McDonald C, McNally EM, Weiss RB, United Dystrophinopathy Project (2012) LTBP4 genotype predicts age of ambulatory loss in Duchenne muscular dystrophy. Ann Neurol 73:481–488
Gehrig SM, Ryall JG, SChertzer JD, Lynch GS (2008) Insulin-like growth factor-I growth factor analogue protects muscle of dystrophic mdx mice from contraction-mediated damage. Exp Physiol 93:1190–1198
Schertzer JD, Gehrig SM, Ryall JG, Lynch GS (2007) Modulation of insulin-like growth factor (IGF)-I and IGF-binding protein interactions enhances skeletal muscle regeneration and ameliorates the dystrophic pathology in mdx mice. Am J Pathol 171:1180–1188
Nigro V, Savarese M (2014) Genetic basis of limb-girdle muscular dystrophies: the 2014 update. Acta Myol 33:1–12
Bogdanovich S, McNally EM, Khurana TS (2008) Myostatin blockade improves function but not histopathology in a murine model of limb-girdle muscular dystrophy 2C. Muscle Nerve 37:308–316
Parsons SA, Millay DP, Sargent MA, McNally EM, Molkentin JD (2006) Age-dependent effect of myostatin blockade on disease severity in a murine model of limb-girdle muscular dystrophy. Am J Pathol 168:1975–1985
Goldstein JA, Kelly SM, LoPresti PP, Heydemann A, Earley JU, Ferguson EL, Wolf MJ, McNally EM (2011) SMAD signaling drives heart and muscle dysfunction in a Drosophila model of muscular dystrophy. Hum Mol Genet 20:894–904
Bartoli M, Poupiot J, Vulin A, Fougerousse F, Arandel L, Daniele N, Roudaut C, Noulet F, Garcia L, Danos O, Richard I (2007) AAV-mediated delivery of a mutated myostatin propeptide ameliorates calpain 3 but not alpha-sarcoglycan deficiency. Gene Ther 14:733–740
Ohsawa Y, Hagiwara H, Nakatani M, Yasue A, Moriyama K, Murakami T, Tsuchida K, Noji S, Sunada Y (2006) Muscular atrophy of caveolin-3-deficient mice is rescued by myostatin inhibition. J Clin Invest 116:2924–2934
Wuebbles RD, Hanel ML, Jones PL (2009) FSHD region gene 1 (FRG1) is crucial for angiogenesis linking FRG1 to facioscapulohumeral muscular dystrophy-associated vasculopathy. Dis Model Mech 2:267–274
Elbaz M, Yanay N, Aga-Mizrachi S, Brunschwig Z, Kassis I, Ettinger K, Barak V, Nevo Y (2012) Losartan, a therapeutic candidate in congenital muscular dystrophy: studies in the dy(2J)/dy(2J) mouse. Ann Neurol 71:699–708
Rosenberg IH (1989) Summary comments. Am J Clin Nutr 50:1231–1233
Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB (1995) Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. New Engl J Med 332:556–561
Hardy SE, Perera S, Roumani YF, Chandler JM, Studenski SA (2007) Improvement in usual gait speed predicts better survival in older adults. J Am Geriatr Soc 55:1727–1734
Dam T-T, Peters KW, Frgala M, Cawthon PM, Harris TB, McLean R, Shardell M, Alley DE, Kenny A, Ferrucci L, Guralnik J, Kiel DP, Kritchevsky S, Vassileva MT, Studenski S (2014) An evidence-based comparison of operational criteria for the presence of sarcopenia. J Gerontol Med A Biol Med Sci 69:584–590
Berardi E, Annibali D, Cassano M, Crippa S, Sampaolesi M (2014) Molecular and cell-based therapies for muscle degenerations: a road under construction. Front Physiol 5:1–13
Wagner KR, Fleckenstein JL, Amato AA, Barhn RJ, Bushby K, Escolar DM, Flanigan KM, Pestronk A, Tawil R, Wolfe GI, Eagle M, Florence JM, King WM, Pandya S, Straub V, Juneau P, Meyers K, Csimma C, Araujo T, Allen R, Parsons SA, Wozney JM, Lavallie ER, Mendell JR (2008) A phase I/II trial of MYO-029 in adult subjects with muscular dystrophy. Ann Neurol 63:561–571
Relizani K, Mouisel E, Giannesini B, Hourde C, Patel K, Gonzales SM, Julich K, Vignaud A, Pietri-Rouxel F, Fortin D, Garcia L, Blot S, Ritvos O, Bendahan D, Ferry A, Ventura-Clapier R, Schuelke M, Amthor H (2014) Blockade of ActRIIB signaling triggers muscle fatigability and metabolic myopathy. Mol Ther 22:1423–1433
Barton ER, Morris L, Musaro A, Rosenthal N, Sweeney HL (2002) Muscle specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J Cell Biol 157:137–148
Schertzer JD, Van Der Poel C, Shavlakadze T, Grounds MD, Lynch GS (2008) Muscle-specific overexpression of IGF-1 improves E–C coupling in skeletal muscle fibers from dystrophic mdx mice. Am J Physiol Cell Physiol 294:C161–C168
Abmayr S, Gregorevic P, Allen JM, Chamberlain JS (2005) Phenotypic improvement of dystrophic muscles by rAAV/microdystrophin vectors is augmented by IGF1 codelivery. Mol Ther 12:441–450
Schertzer JD, Gehrig SM, Ryall JG, Lynch GS (2007) Modulation of insulin-like growth factor (IGF)-I and IGF-binding protein interactions enhances skeletal muscle regeneration and ameliorates the dystrophic pathology in mdx mice. Am J Pathol 171:1180–1188
Burks TN, Cohn RD (2011) Role of TGF-β signaling in inherited and acquired myopathies. Skelet Muscle 1:19
Yamazaki M, Minota S, Sakurai H, Miyazono K, Yamada A, Kanazawa I, Kawai M (1994) Expression of transforming growth factor-beta 1 and its relation to endomysial fibrosis in progressive muscular dystrophy. Am J Pathol 144:221–226
Bernasconi P, Torchiana E, Confalonieri P, Brugnoni R, Barresi R, Mora M, Cornelio F, Morandi L, Mantegazza R (1995) Expression of transforming growth factor-beta 1 in dystrophic patient muscles correlates with fibrosis. Pathogenetic role of a fibrogenic cytokine. J Clin Invest 96:1137–1144
Kolodziejczyk SM, Walsh GS, Balazsi K, Seale P, Sandoz J, Hierlihy AM, Rudnicki MA, Chamberlain JS, Miller FD, Megeney LA (2001) Activation of JNK1 contributes to dystrophic muscle pathogenesis. Curr Biol 11:1278–1282
Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MC, Gamradt M, Ap Rhys CM, Holm TM, Loeys BL, Ramirez F, Judge DP, Ward CW, Dietz HC (2007) Angiotensin II type 1 receptor blockade attenuates TGF-geat-induced failure of muscle regeneration in multiple myopathic states. Nat Med 13:204–210
Meinen S, Lin S, Ruegg MA (2012) Angiotensin II type 1 receptor anatagonists alleviate muscle pathology in the mouse model for laminin-α2-deficient congenital muscular dystrophy (MDC1A). Skelet Muscle 2:18
Hermans MCE, Pinto YM, Merkies LSJ, de Die-Smulders CEM, Crijns HJGM, Faber CG (2010) Hereditary muscular dystrophies and the heart. Neuromusc Disord 20:479–492
Shin JH, Nitahara-Kasahara Y, Hayashita-Kinoh H, Ohshima-Hosoyama S, Kinoshita K, Chiyo T, Okada H, Okada T, Takeda S (2011) Improvement of cardiac fibrosis in dystrophic mice by rAAV9-mediated microdystrophin transduction. Gene Ther 18(9):910–919
Yue Y, Li Z, Harper SQ, Davisson RL, Chamberlain JS, Duan D (2003) Microdystrophin gene therapy of cardiomyopathy restores dystrophin-glycoprotein complex and improves sarcolemma integrity in the mdx mouse heart. Circulation 108(13):1626–1632
Wu B, Moulton HM, Iversen PL, Jiang J, Li J, Li J, Spurney CF, Sali A, Guerron AD, Nagaraju K, Doran T, Lu P, Xiao X, Lu QL (2008) Effective rescue of dystrophin improves cardiac function in dystrophin-deficient mice by a modified morpholino oligomer. Proc Natl Acad Sci USA 105(39):14814–14819
Spurney CF, Sali A, Guerron AD, Iantorno M, Yu Q, Gordish-Dressman H, Rayavarapu S, van der Meulen J, Hoffman EP, Nagaraju K (2011) Losartan decreases cardiac muscle fibrosis and improves cardiac function in dystrophin-deficient mdx mice. J Cardiovasc Pharmacol Ther 16(1):87–95
Frontera WR, Hughes VA, Krivickas LS, Kim SK, Foldvari M, Roubenoff R (2003) Strength training in older women: early and late changes in whole muscle and single cells. Muscle Nerve 28:601–608
Schroeder ET, He J, Yarasheski KE, Binder EF, Castaneda-Sceppa C, Bhasin S, Dieli-Conwright CM, Kawakubo M, Roubenoff R, Azen SP, Sattler FR (2012) Value of measuring muscle performance to assess changes in lean mass with testosterone and growth hormone supplementation. Eur J Appl Physiol 112:1123–1131
Conflict of Interest
Ronenn Roubenoff and Matthew N. Meriggioli are employees of Novartis Institutes for Biomedical Research, which conducts research and development of treatments for muscle wasting.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meriggioli, M.N., Roubenoff, R. Prospect for Pharmacological Therapies to Treat Skeletal Muscle Dysfunction. Calcif Tissue Int 96, 234–242 (2015). https://doi.org/10.1007/s00223-014-9926-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-014-9926-8