Abstract
Purpose
The purpose of this study was to investigate if a 2-year intervention with a minimal resource fracture liaison service (FLS) was associated with increased investigation and medical treatment and if treatment was related to reduced re-fracture risk.
Methods
The FLS started in 2013 using existing secretaries (without an FLS coordinator) at the emergency department and orthopaedic wards to identify risk patients. All patients older than 50 years of age with a fractured hip, vertebra, shoulder, wrist or pelvis were followed during 2013–2014 (n = 2713) and compared with their historic counterparts in 2011–2012 (n = 2616) at the same hospital. Re-fractures were X-ray verified. A time-dependent adjusted (for age, sex, previous fracture, index fracture type, prevalent treatment, comorbidity and secondary osteoporosis) Cox model was used.
Results
The minimal resource FLS increased the proportion of DXA-investigated patients after fracture from 7.6 to 39.6 % (p < 0.001) and the treatment rate after fracture from 12.6 to 31.8 %, which is well in line with FLS types using the conventional coordinator model. Treated patients had a 51 % lower risk of any re-fracture than untreated patients (HR 0.49, 95 % CI 0.37–0.65 p < 0.001).
Conclusions
We found that our minimal resource FLS was effective in increasing investigation and treatment, in line with conventional coordinator-based services, and that treated patients had a 51 % reduced risk of new fractures, indicating that also non-coordinator based fracture liaison services can improve secondary prevention of fractures.
Similar content being viewed by others
Introduction
In Sweden, the fourth most important cause of illness is fall-related injuries [1]. Falls often cause fractures, and the yearly fracture-related cost of osteoporosis in Sweden has been estimated to be €0.5 billion [2]. Fractures are common and dangerous. At the age of 50, the risk of sustaining a fragility fracture (i.e. a fracture caused by an injury that would be insufficient to fracture a normal bone) during the remaining lifetime is 50 % for women and 20 % for men [3]. Fragility fractures are associated with an increased morbidity and mortality, especially hip and vertebral fractures in which the risk of mortality is twice as high for patients older than 80 years compared to age-matched controls [4].
There is solid evidence that medical treatment of osteoporosis reduces fracture rate in postmenopausal women, especially with low BMD and high fracture risk [5]. Alendronate treatment leads to considerable relative risk reduction (RRR), 45 % for vertebral fractures and 40 % for hip fractures [6]. With a yearly dose of zoledronic acid, the RRR is even higher, 70 % for vertebral fractures and 41 % for hip fractures [7]. Denosumab treatment has also proven effective to reduce fracture risk. For women older than 75 years or with T-score <2.5 SD, the RRR for a hip fracture was 61 %, and for patients with multiple vertebral fractures, the RRR for subsequent vertebral fracture was 55 % [8].
A patient with a prior fracture has an increased risk of a new fracture [9]. For most fracture sites, the risk is doubled, but a patient with a vertebral fracture has a 4-fold increased risk of a new vertebral fracture [9]. In spite of well-documented evidence for fracture preventing medication, only a minority receive medical treatment within 6–12 months after an osteoporotic fracture (14 % of Swedish women and 3 % of men, aged 50 years or more) [10].
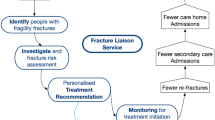
In an effort to increase the proportion of treated patients after a fracture, structured prevention programs (fracture liaison services, FLS) have been introduced to identify, investigate and reduce patients at risk of new fractures. Demonstrably, FLS programs show an increase in the number of patients investigated and treated [11, 12].
However, there are few studies attempting to report on the FLS main objective, which is prevention of new fractures. Furthermore, previously reported studies may raise quality concerns [13–17]. Two studies created a control group from the patients who chose not to participate in the FLS [13, 15]. Two other studies used another hospital without an FLS as a control [16, 17]. Both approaches raise selection bias issues. Three of the studies used questionnaires to record re-fractures [13–15]. However, self-reported fractures have been associated with 11–29 % false positives [18–20] and 7 % false negatives [19]. Thus, using this method to record fractures introduces an error of the same magnitude as the expected effect from treatment [6]. The previously reported studies only included between 41 and 225 re-fractures.
The gold standard when introducing an FLS is to use a coordinator to select patients and manage the patient flow [21]. However, this would require specific additional funds that may not be available at all hospitals. Therefore, we investigated an FLS without a coordinator, introduced with minimal organisational changes. The aim of the performed study was to investigate if a minimal effort FLS (2-year intervention) was associated with increased investigation and medical treatment in a large cohort (n = 5,329) of men and women with fracture.
Materials and methods
Fracture liaison service
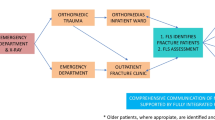
Skaraborg Hospital, a Swedish regional hospital covering 265,000 inhabitants, started an FLS on 1 January 2013 and included all patients older than 50 years seeking care for an osteoporotic fracture (defined as a fracture of the wrist, upper arm, hip, vertebra or pelvis). The FLS comprised four steps.
-
1.
Risk patient identification: A total of ∼30 secretaries at the emergency department and the orthopaedic clinic were responsible for identifying risk patients and order a bone density measurement (DXA) and fracture risk (FRAX) assessment based on ICD-10 fracture codes in the patient charts. Patients with pathological fractures or who were deceased prior to DXA referral were excluded.
-
2.
DXA examination and FRAX assessment: Patients were invited to the hospital’s DXA unit where experienced nurses performed the DXA examination as well as a FRAX assessment.
-
3.
DXA and FRAX evaluation: Based on regional and national guidelines [22], an experienced endocrinology consultant assessed the bone densitometry results and clinical risk factors, defined a diagnosis and provided treatment recommendation and follow-up advice, which were sent to the patient’s primary care physician.
-
4.
Treatment initiation: Based on the recommendation and in consultation with the patient, the primary care physician initiated treatment in order to reduce the risk for a new fracture. The primary care physician was responsible to rule out secondary osteoporosis and choose a suitable type of osteoporosis medication.
Our FLS model had two key elements: the delegation of referral power to secretaries and the detailed consultant response, the former to enable organisational stability and robustness and the latter to reduce the workload for the receiving doctor.
During the first year, 2013, patients’ relatives or staff at nursing homes often cancelled the examination due to perceived high patient age or morbidity. This leads to a relatively high DXA cancellation rate among the elderly, 50 % for patients over 80 years compared to 23 % for 50–79 years (p < 0.001). By cancelling the DXA, the patient also lost the possibility of being eligible for treatment through the FLS. Therefore, starting January 2014, the hospital decided to offer treatment with a yearly intravenous infusion of zoledronic acid (5 mg) to all patients older than 80 years with a hip fracture, without prior DXA examination.
Study design
The potential FLS effect was investigated comparing the intervention period, 2013–2014, with the control period, 2011–2012. All patients older than 50 years with an index osteoporotic fracture, the types targeted by the FLS, were included in the study. Patients with the selected fracture types represented 56 % of all fractured patients. The intervention group consisted of patients presenting with an osteoporotic fracture during 2013–2014 (n = 2616), i.e. when the FLS had started. The control group consisted of historical controls 2011–2012 (n = 2713). The follow-up time varied from 0 to 2 years, i.e. each period was truncated at 31 December 2012 and 2014 respectively in order to achieve group symmetry and to avoid crossover effects. In order to assess potential fracture risk reduction from medical treatment, all treated patients, regardless of period, were compared with the non-treated patients. Subgroup analysis was performed on hip fracture patients above 80 years of age since they were handled slightly differently in the process.
Definition of variables
In this study, the term fracture refers to a fracture event, i.e. one or more fractures at the same occasion, with an ICD-10 diagnosis that starts with S12, S22, S32, S42, S52, S62, S72, S82, S92, T02, T08, T10, T12, T142 or M485. If a secretary sent a referral for DXA as instructed, it was automatically registered in the digitalized patient record. The age was set at the time of the index fracture, and gender was derived from the patient’s social security number.
The follow-up time in the study was calculated from the fracture date (or if the patient was admitted, from the date of discharge) to the actual event, where event refers to DXA scan, medical treatment, a subsequent fracture or death, depending on analysed outcome. The follow-up time was truncated if a patient moved from the region, died or if the follow-up period ended.
Previous fracture was defined as any other fracture before the date of the index fracture, stretching exactly 14 years back in time.
Three types of prevalent medications were accounted for: (i) active medical treatment for osteoporosis, (ii) calcium and/or vitamin D and (iii) glucocorticoids. Prevalent active medical treatment for osteoporosis was included as number of days during the year prior to fracture with bisphosphonates, denosumab, strontium ranelate or hormonal treatment (p.o. or transdermal). Similarly, a separate variable was calculated for calcium and/or vitamin D. Prevalent glucocorticoid treatment was defined as milligrams of prednisolone equivalents during the year prior to fracture. The Prednisolone equivalents were calculated using conversion factors: betamethasone (8.33), dexamethasone (6.67), methylprednisolone (1.25), hydrocortisone (0.25) and cortisone (0.2).
Incident medical treatment was defined as number of days of treatment, which started at the first date of medication retrieval from the pharmacy after the index fracture and ended when prescription ended, a subsequent fracture or death occurred or if the patient moved out of the area. For prescription medication, treatment refers to the patient retrieving medication at the pharmacy whereas parenteral treatment administered at the hospital was registered in the patient’s record.
Categorical variables were created for prevalent illnesses, if the ICD-10 diagnosis was present in the hospital records within 10 years prior to index fracture. The illnesses included osteoporosis with or without fracture, rheumatoid arthritis and all diagnoses with prevalence higher than 5 % that might correlate with fracture risk. We also created a variable for secondary osteoporosis according to FRAX and all its components (insulin-dependent diabetes, hyperthyroidism, hypogonadism, malnutrition, osteogenesis imperfecta or chronic liver disease). A modified Charlson comorbidity index was used to summarize and quantify comorbidity. The index was calculated as a sum of the following diseases, ICD-10 codes in parenthesis: one point each for an ischaemic heart disease (I20–I25); congestive heart failure (I50); cerebrovascular disease (I60–I69); disease of arteries, arterioles and capillaries (I70–I79); diabetes (E10–14); dementia (F00–F03); chronic pulmonary disease (J43–J46); and chronic liver disease (parts of K70–K77); two points each for renal failure (N17–N19), hemiplegia (G81); lymphoma or leukemia (C81–C96); and tumor without metastasis (C00–C76, C80, C97, 5-year history); and six points for metastatic solid tumor (C77–C79)—10-year history if not stated otherwise.
Statistics
Chi-square tests were used on categorical variables in order to investigate statistical differences between the control and intervention groups in terms of comorbidity, previous medications and known risk factors. Differences between the continuous variables were investigated using t test. A Cox proportional hazard regression model was used for time-dependent analysis. When DXA or treatment was the outcome, adjustment was made only for age and gender (crude). When analysing risk of new fracture or death, adjustment was also made for previous fracture, index fracture type (hip fracture, vertebral fracture or other), prevalent medical treatment, prevalent calcium or vitamin D treatment, prevalent glucocorticoid treatment, prevalent osteoporosis diagnosis, rheumatoid arthritis and secondary osteoporosis according to FRAX as well as a modified Charlson morbidity index (fully adjusted). A p value less than 0.05 was considered significant.
Statistical power calculation
The primary aim was to study the FLS effect on DXA utilization and treatment rates. Assuming 1500 osteoporotic fractures occur in Skaraborg per year, the mean follow-up time in this 2-year study is 1 year (different starting points, fixed end-point). Approximately 14 % receive treatment after fracture [10]. Assuming an alpha-value of 0.05, any postintervention treatment rate above 16 % would render a power above 80 %. Since DXA measurements in most cases precede treatment, we expect to find similar or larger increases in DXA utilization and therefore increased statistical power for this end-point.
Regarding re-fractures in approximately 3000 patients in each group, mean follow-up time of 1 year, assuming an alpha-value of 0.05, an annual 8 % re-fracture rate without treatment, an increase from 14 to 50 % in treatment rates and a 50 % reduction of re-fractures from treatment would render a statistical power of 82 %. However, assuming a 4-month average delay from index fracture to start of treatment, the statistical power falls to 51 % for the re-fracture end-point.
Assessment of BMD
Assessment of BMD (aBMD, g/cm2) was measured at the hip, femoral neck and lumbar spine (L1–L4) using a Lunar Prodigy (GE Healthcare). The same DXA machine was used for all measurements. The coefficients of variations (CV) ranged from 0.7 to 1.5 %.
Information sources
The hospital’s digitalized patient records were used to identify patients with fractures during 2011–2014. In order to differ records of re-visits regarding an old fracture from records of new fractures, manual verification was performed. If a patient had fracture entries on more than one occasion, that patient’s hospital record was reviewed and only entries representing true fracture occasions were kept. Thus, all re-fractures were manually verified in the patient’s hospital records.
Information on the identified cohort concerning previous fractures, current and previous diseases and medication as well as date of DXA examination was also collected from the hospital’s patient records. Date of death and moving in or out of the hospital’s district was collected from the Swedish Population Registry.
Results
Patient characteristics
A total of 5329 patients were included in the study, with 2616 in the intervention group and 2713 in the control group. The accumulated follow-up time was 5014 years, with 2481 years in the intervention group and 2534 years in the control group. The mean follow-up time (mean ± SD) was 344 days (346 ± 219 days in the intervention versus 341 ± 221 days in the control group, p = 0.39).
Baseline characteristics
Baseline characteristics are presented in Table 1. Apart for slightly less patients with hypertension in the intervention group, there were no significant differences between the intervention group and the control group with regard to sex, age, proportion of previous fracture, prevalence of osteoporosis, rheumatoid arthritis, secondary osteoporosis according to FRAX, modified Charlson comorbidity index, other possible fall- or fracture-related diseases with prevalence above 5 % or type of index fracture (Table 1).
In the subgroup hip fracture patients above 80 years, the intervention group was slightly less inclined to have previous glucocorticoid treatment, but otherwise no significant differences were observed (Table 1).
In the group selected for treatment, the prevalence of previous fracture was higher, as well as the proportion of women and vertebral index fracture, than in the untreated group (Table 1). Patients selected for treatment were slightly healthier according to Charlson (Table 1) and less prone to have dementia (2.1 versus 4.0 %, p < 0.01) than their untreated counterparts were. Also, patients selected for treatment were more inclined to already have treatment for osteoporosis as well as glucocorticoid treatment (Table 1).
FLS patient identification
Of all the osteoporotic fractures in 2013–2014, 11 % were omitted by the system, i.e. occurring in a unit with no FLS secretary. Of the patients treated at an FLS unit, the secretaries identified 75 % of the defined osteoporotic fractures. Admitted patients had a higher likelihood of being included than did patients visiting the emergency room (88 versus 61 %, p < 0.001). The likelihood of being included also differed by index fracture type: hip 92 %, vertebral 60 %, wrist 76 %, shoulder 55 % and pelvic 67 % (p < 0.001).
Increased likelihood of DXA examination
The proportion of patients being examined with DXA after a fracture increased from 7.6 % in the control period to 39.6 % in the intervention period (p < 0.001). The time to DXA examination (mean ± SD) after index fracture decreased from 133 ± 119 to 85 ± 67 days (p < 0.001), and the patients examined had no statistical difference in terms of femoral neck BMD (0.72 ± 0.13 versus 0.73 ± 0.13, p = 0.36). Age- and sex-adjusted time-dependent Cox regression analysis of the intervention period versus the control period showed a 7-fold increased likelihood of being examined with a DXA (HR 7.0, 95 % CI 6.0–8.1 p < 0.001) in the intervention period (Fig. 1a). These results were highly similar when fully adjusted (HR 7.1, 95 % CI 6.1–8.2, p < 0.001).
Increased likelihood of medical treatment
There was no significant difference in prevalent osteoporosis treatment rates between the control (9.2 %) and the intervention period (9.7 %). While the treatment rate after a fracture only increased to 12.6 % (n = 341) in the control period, it increased to 31.8 % (n = 833) in the intervention period (p < 0.001). Excluding patients with previous treatment, there was no significant difference in time to treatment (mean ± SD) in the control period (123 ± 115 days) compared to the intervention period (118 ± 79), p = 0.33. Age- and sex-adjusted time-dependent Cox regression analysis of the intervention period versus the control period revealed a 2.8-fold increased likelihood of being treated (HR 2.8, 95 % CI 2.5–3.2, p < 0.001, Fig. 1b). These results were highly similar when fully adjusted (HR 2.6, 95 % CI 2.3–2.9, p < 0.001). A breakdown of types of treatment showed that the proportion of zoledronic acid and denosumab increased dramatically in the intervention group (Table 2).
Re-fractures
There were a total of 469 new fractures in 444 patients, and the most common re-fracture site was the hip (Table 3). There was no significant difference in re-fracture rate in the control period compared to the intervention period—228 (8.4 %) versus 216 (8.3 %), p = 0.85. Time to new fracture (mean ± SD) was 207 ± 168 days in the control and 200 ± 163 in the intervention period, p = 0.66. Using a time-dependent Cox analysis, the risk reduction was also non-significant (crude HR 0.95, 95 % CI 0.79–1.14, p = 0.59, and fully adjusted HR 0.95, 95 % CI 0.79–1.14, p = 0.60).
Deaths
There was no significant difference in mortality rate in the control period compared to the intervention period: 361 (13.3 %) versus 320 (12.2 %), p = 0.24. Time to death (mean ± SD) was 178 ± 169 days in the control and 186 ± 162 in the intervention period, p = 0.56. A time-dependent Cox analysis showed that the mortality in the intervention group tended to be lower, but the reduction was not statistically significant (crude HR 0.87, 95 % CI 0.75–1.01, p = 0.07, and fully adjusted HR 0.88, 95 % CI 0.76–1.03, p = 0.11).
Subgroup analysis
In hip fracture patients older than 80 years, the ratio of DXA measurement after index fracture increased from 1.0 to 26.1 % or using a Cox proportional hazard analysis adjusted for age and sex, which is a 32-fold increased likelihood (HR 32.4, 95 % CI 14.3–73.3, p < 0.001).
The chance of receiving medical treatment increased from 8.4 to 28.3 %, or using Cox proportional hazard analysis adjusted for age and sex, which is a 3.9-fold increased likelihood (HR 3.9, 95 % CI 2.8–5.4, p < 0.001). Just like for the whole group, when excluding patients with previous treatment, there was no difference in time to treatment (mean ± SD), 127 ± 119 days in the control versus 105 ± 47 days in the intervention group, p = 0.24.
Regarding re-fractures, there was no significant difference in time to new fracture, 174 ± 168 days versus 160 ± 152 days (p = 0.66), but a significant reduction in re-fracture rate from 11.9 to 7.8 % (p = 0.02) was observed comparing the control and FLS periods. Using the time-dependent Cox model adjusted for age and sex, the FLS period was associated with a 35 % reduction of re-fracture rate (HR 0.65, 95 % CI 0.45–0.95, p = 0.03). This reduction was similar (HR 0.65 95 % CI 0.44–0–94, p = 0.02) when fully adjusted.
Treated patients had reduced risk of re-fracture
Regardless of period, the treated patients had a significantly lower fracture rate versus the non-treated patients (6.6 versus 8.8 %, p = 0.02) as well as longer mean time to new fracture, 294 days versus 185 days (p < 0.001). Using a time-dependent Cox model, treated patients had a 40 % reduction of risk for new fracture (crude HR 0.60, 95 % CI 0.47–0.77, p < 0.001) or 51 % after adjustments for covariates (HR 0.49, 95 % CI 0.37–0.65, p < 0.001, Fig. 2) than did untreated patients.
Using a fully adjusted Cox analysis, the risk reduction of specific future fractures was even higher: for hip fractures 64 % (p < 0.01), for vertebral fracture 73 % (p < 0.01), for major osteoporotic fractures 67 % (p < 0.001) and for patients with multiple future fracture events 63 % (p = 0.047). In the subgroup of patients older than 80 years, the risk reduction was slightly higher than for the treated group as a whole, 54 % (p < 0.001) (Table 4).
Mortality
The association between the Charlson comorbidity index and mortality was analysed using a time-dependent Cox regression, adjusted for sex and age with death as outcome. For each increased point in Charlson, the mortality was increased by 30 % (HR 1.30, 95 % CI 1.21–1.39, p < 0.001). The mortality rate was significantly lower in the treated group compared to the untreated group (6.4 versus 14.5 %, p < 0.001), and the mean time to death was longer in the treated group compared to the untreated group (244 versus 175 days, p < 0.01). Time-dependent Cox analysis showed that the treated group had a 56 % (crude HR 0.44, 95 % CI 0.34–0.56, p < 0.001) or 66 % (fully adjusted HR 0.34, 95 % CI 0.25–0.44, p < 0.001) lower risk of mortality than did untreated patients.
Discussion
This study shows a remarkable 7-fold increase in patients receiving DXA examination as well as a 2.8-fold increased chance of receiving pharmacological osteoporosis treatment as a result of a minimal resource (non-coordinator-based) FLS. Patients receiving osteoporosis medication had a 51 % lower re-fracture rate. With a re-fracture rate of 8.8 % for the untreated, treatment was associated with an absolute risk reduction (ARR) of 4.5 %, which translates to 66 prevented fractures per year in our total population sustaining a total of 1332 fractures yearly.
The reduction of re-fracture risk in the intervention group was not significant for the study as a whole, but in the subgroup of hip fracture patients older than 80 years, the FLS was associated with a 35 % significantly lower re-fracture risk. During the second year (2014) the FLS was used, these patients were able to receive zoledronic acid treatment (at the hospital or in their homes) without prior DXA assessment, which increased the proportion receiving treatment even further. FLS patients in this category had a lower proportion of prevalent glucocorticoid treatment, but were similar to controls in regard to comorbidity, prevalent fracture and other medical treatments. Even though the FLS was associated with a reduced re-fracture rate in Cox models adjusted for glucocorticoid use and other covariates, it cannot be ruled out that the selection of patients contributed to the association. A post hoc power analysis based on actual risk reduction from 11.9 to 7.8 % using an alpha-value of 5 % revealed a statistical power of 89 % in this particular subgroup.
The mortality rate among the patients with a re-fracture was 16.6 versus 12.5 % among patients without a re-fracture, indicating a relationship between re-fracture and mortality. However, when comparing the treated patients with the untreated, the reduction in mortality (66 %) was greater than the reduction in re-fracture (51 %), even after adjustment for sex, age, comorbidity, previous illnesses and medication. As expected, the Charlson comorbidity index was associated with mortality. However, the large mortality risk reduction in patients selected for medical treatment suggests that treated patients were healthier, although we could not account for this using our available data. In a meta-analysis of randomized controlled trials, osteoporosis treatment has been shown to reduce mortality by 11 % [23].
In our hospital, there is no specific department for osteoporosis patients, which is partly why the distributed secretary-based model was chosen instead of a central coordinator-based model, which would have been more exposed to organisational changes and required additional staff. If all types of fractures would have been included in the FLS, we speculate that the secretaries’ ability to recall and identify patients to refer to DXA would increase, which could possibly have reduced the 25 % omitting rate.
To compare our minimal resource FLS with a conventional coordinator-based FLS, the only procedure that differs is patient identification. Even though our FLS required no additional staff, our model required an estimated 5 min of extra secretarial time per patient, both for identification and investigating if the patient was recently examined. Altogether, the estimated additional time spent by the secretaries to carry out these tasks amounted to approximately ten work days per year.
Since the effect of medication on fracture risk is undisputed, it seems unethical to design randomized studies for evaluation of FLS programs. Ganda et al. [12] summarised the evidence of the effectiveness of different FLS and graded them depending on the degree of involvement: if an osteoporosis unit both investigated and treated (type A), if an osteoporosis unit investigated but sent treatment recommendations to the primary care physician (type B), if the hospital informed the primary care physician of the fracture episode (type C) or if the hospital informed the patient of being at risk for a re-fracture and should become investigated (type D). Our FLS mostly resembled a type B FLS, but since a proportion of patients were treated by the hospital staff, the FLS to some extent resembled a type A service. Type B FLS models were able to increase DXA utilization from 9 to 59 % [12]. Our minimal resource FLS increased the utilization rates from 8 to 40 %. However, the follow-up data of the late participants in the study were truncated at the end of the period. If only looking at 2011 participants versus those in 2013, the increase was larger, from 9 to 50 % (p < 0.001), thus almost as good as a typical type B model. When comparing the ability to get patients to treatment, our minimal effort FLS increased from 13 % (2011–2012) to 32 % (2013–2014) (p < 0.001) or from 14 % (2011) to 39 % (2013) (p < 0.001). This is an increase well in line with the type B FLS increase from 20 to 41 % (p = 0.01) and almost as efficient as a type A service, increasing treatment from 18 to 46 % (p < 0.001) [12].
In 2008, Dell et al. described the Kaiser Southern California Healthy Bones Program, a comprehensive program including 620,000 patients, and showed a 37 % average reduction in hip fracture rate from 2006 versus an expected rate based on age-adjusted hip fracture rates from 1997 to 1999 [24]. Lih et al. [13] described a 4-year prospective study with 1544 patients in Sydney, Australia, and reported an 80 % reduction of risk of further fracture. Controls, however, were chosen from the group that chose not to attend the FLS, which introduces potential selection bias issues. As opposed to Lih et al., we used historical controls. In Lund, Sweden, Åstrand et al. [14] reported a 42 % risk reduction in a screened group in a 6-year follow-up of 592 fracture patients 50–75 years of age with a major osteoporotic fracture. However, a time-dependent Cox model was not used and there were cross-over effects from the control to the intervention group. Furthermore, a questionnaire was used to register re-fractures, with a response rate of 77 %, and self-reported fractures are known to be associated with uncertainty [18–20]. In the present study, we used a time-dependent Cox model, truncated the follow-up in order to avoid cross-over effects and used manually verified X-ray records for re-fracture reporting.
Huntjens et al. [16] in the Netherlands compared patients with non-vertebral fractures presenting at a hospital with FLS (n = 1412) with ditto patients at a neighbouring hospital without FLS (n = 1910). There was no difference in re-fracture rate after 2 years (6.7 versus 6.8 %), but a time-dependent Cox model showed a 56 % reduction of non-vertebral fractures and a 35 % reduction of mortality, but the result could have been affected by the differences in population demographics between the two hospitals. Van Der Kallen et al. [15] compared, in a 2-year follow-up, 214 FLS patients with 220 patients who had chosen not to participate in the FLS, did not adjust for mortality and used a questionnaire to report rate of re-fracture and found a reduction of fracture rate from 16.4 to 5.1 %. Choosing non-participants as controls exposes the result for a potential selection bias.
In 2015, Nakayama et al. reported a 30 % reduction in re-fracture rate over a 3-year follow-up period when comparing an FLS hospital with a non-FLS hospital [17]. However, the hospitals had different sizes and treated somewhat different patient groups, indicating possible problems of selection bias. Furthermore, the fact that the number of re-fractures was quite few suggests inadequate statistical power. Our study was performed in the same hospital with historical controls. Apart from Nakayama’s study, the above studies [13–16, 24] included patients actually participating in an FLS program whereas our study included all potential patients being eligible and compared data from the FLS period with a historic period, minimizing the risk of selection bias. In our study, 68 % of the patients offered participation accepted a DXA examination and/or medication.
Lih et al. showed that the FLS association with re-fracture rate remained significant in the elderly when calculating separately for patients above and below 70 years of age [13]. Our study demonstrates that osteoporosis treatment was associated with equal or greater relative risk reduction in patients older than 80 years of age than in all treated patients in the study, 54 versus 51 %.
Thus, several previous studies [13–15, 17, 24] have reported that an FLS is associated with reduced fracture rates, but many studies suffer from different quality issues and lack power calculations. Using a post hoc power analysis on reported re-fracture rate in the control versus the intervention group, most studies [13–15, 17] appear to have sufficient power (73–100 %). However, even with high power, the results could be affected by quality issues such as uncertainty regarding self-reported re-fractures, differences in demographics between control and intervention hospitals or selection bias when using no-shows as control. Therefore, it could be of interest to reassess the post hoc power analysis, but instead use a prediction model for re-fracture rates in the FLS. Assuming that the re-fracture rates of the control group were true, the re-fracture rates of the intervention group can be calculated based on reported treatment rates (if not reported, 10 % in the control group versus 50 % in the FLS were assumed) and a 50 % fracture risk reduction from treatment. Using these assumptions, the statistical power of the previous studies would range from 7 to 54 % [13–17], while our study reaches 51 %. Assuming a 4-month delay before the patient is likely to receive treatment after index fracture resulted in further reduced statistical power to between 7 and 39 % for the previous studies and to 24 % in our study. Persistence rates are not included in the model, which are known to be low, around 50 % [25], which would decrease the difference in re-fracture rate and reduce the expected power even further. Thus, all yet existing studies investigating re-fracture reduction by FLS, including the present study, appear to be underpowered.
The present study investigating a minimal resource FLS, implemented using already existing staff, clearly demonstrates large effects in increasing DXA investigation and treatment rates. Among the strengths of our study is the fact that it covers a large patient group (n = 5329) with comprehensive background data on comorbidities and medications. The manual verification of the registers using patient charts and X-ray reports strengthens the quality of re-fracture data and offers a reliable re-fracture rate. Also, clinical vertebral fractures, often omitted in other studies, were included in our study. Furthermore, our control group is clearly defined with the same patient category from the same hospital and patient preference was not an inclusion factor.
Conclusions
We conclude that a minimal resource FLS increased the number of fracture patients being DXA-examined and treated, resembling increases observed in coordinator-based FLS models. Patients receiving osteoporosis medication had reduced risk of any major osteoporotic, vertebral and hip fracture. Furthermore, the studied FLS was related to reduced re-fracture rate in hip fracture patients 80 years or older, but this result could have been affected by insufficient statistical power in the analysis.
References
Murray CJ, Barber RM, Foreman KJ et al (2015) Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet (Lond, Engl). doi:10.1016/s0140-6736(15)61340-x
Borgstrom F, Zethraeus N, Johnell O, Lidgren L, Ponzer S, Svensson O, Abdon P, Ornstein E, Lunsjo K, Thorngren KG, Sernbo I, Rehnberg C, Jonsson B (2006) Costs and quality of life associated with osteoporosis-related fractures in Sweden. Osteoporos Int 17(5):637–650. doi:10.1007/s00198-005-0015-8
Sambrook P, Cooper C (2006) Osteoporosis. Lancet 367(9527):2010–2018. doi:10.1016/s0140-6736(06)68891-0
Sattui SE, Saag KG (2014) Fracture mortality: associations with epidemiology and osteoporosis treatment. Nat Rev Endocrinol 10(10):592–602. doi:10.1038/nrendo.2014.125
Lorentzon M, Cummings SR (2015) Osteoporosis: the evolution of a diagnosis. J Intern Med 277(6):650–661. doi:10.1111/joim.12369
Wells George A, Cranney A, Peterson J, Boucher M, Shea B, Welch V, Coyle D, Tugwell P (2008) Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev (1). doi:10.1002/14651858.CD001155.pub2
Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356(18):1809–1822. doi:10.1056/NEJMoa067312
Boonen S, Adachi JD, Man Z, Cummings SR, Lippuner K, Torring O, Gallagher JC, Farrerons J, Wang A, Franchimont N, San Martin J, Grauer A, McClung M (2011) Treatment with denosumab reduces the incidence of new vertebral and hip fractures in postmenopausal women at high risk. J Clin Endocrinol Metab 96(6):1727–1736. doi:10.1210/jc.2010-2784
Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, Berger M (2000) Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 15(4):721–739. doi:10.1359/jbmr.2000.15.4.721
Quality and Efficiency in Swedish Health Care - Regional Comparisons 2013 by the Swedish Association of Local Authorities and Regions (2013)
Sale JE, Beaton D, Posen J, Elliot-Gibson V, Bogoch E (2011) Systematic review on interventions to improve osteoporosis investigation and treatment in fragility fracture patients. Osteoporos Int 22(7):2067–2082. doi:10.1007/s00198-011-1544-y
Ganda K, Puech M, Chen JS, Speerin R, Bleasel J, Center JR, Eisman JA, March L, Seibel MJ (2013) Models of care for the secondary prevention of osteoporotic fractures: a systematic review and meta-analysis. Osteoporos Int 24(2):393–406. doi:10.1007/s00198-012-2090-y
Lih A, Nandapalan H, Kim M, Yap C, Lee P, Ganda K, Seibel MJ (2011) Targeted intervention reduces refracture rates in patients with incident non-vertebral osteoporotic fractures: a 4-year prospective controlled study. Osteoporos Int 22(3):849–858. doi:10.1007/s00198-010-1477-x
Astrand J, Nilsson J, Thorngren KG (2012) Screening for osteoporosis reduced new fracture incidence by almost half: a 6-year follow-up of 592 fracture patients from an osteoporosis screening program. Acta Orthop 83(6):661–665. doi:10.3109/17453674.2012.747922
Van der Kallen J, Giles M, Cooper K, Gill K, Parker V, Tembo A, Major G, Ross L, Carter J (2014) A fracture prevention service reduces further fractures two years after incident minimal trauma fracture. Int J Rheum Dis 17(2):195–203. doi:10.1111/1756-185X.12101
Huntjens KM, van Geel TA, van den Bergh JP, van Helden S, Willems P, Winkens B, Eisman JA, Geusens PP, Brink PR (2014) Fracture liaison service: impact on subsequent nonvertebral fracture incidence and mortality. J Bone Joint Surg Am 96(4):e29. doi:10.2106/JBJS.L.00223
Nakayama A, Major G, Holliday E, Attia J, Bogduk N (2015) Evidence of effectiveness of a fracture liaison service to reduce the re-fracture rate. Osteoporos Int. doi:10.1007/s00198-015-3443-0
Chen Z, Kooperberg C, Pettinger MB, Bassford T, Cauley JA, LaCroix AZ, Lewis CE, Kipersztok S, Borne C, Jackson RD (2004) Validity of self-report for fractures among a multiethnic cohort of postmenopausal women: results from the Womenʼs Health Initiative observational study and clinical trials. Menopause 11(3):264–274. doi:10.1097/01.gme.0000094210.15096.fd
Ismail AA, O’Neill TW, Cockerill W, Finn JD, Cannata JB, Hoszowski K, Johnell O, Matthis C, Raspe H, Raspe A, Reeve J, Silman AJ (2000) Validity of self-report of fractures: results from a prospective study in men and women across Europe. EPOS Study Group. European Prospective Osteoporosis Study Group. Osteoporos Int 11(3):248–254
Nevitt MC, Cummings SR, Browner WS, Seeley DG, Cauley JA, Vogt TM, Black DM (1992) The accuracy of self-report of fractures in elderly women: evidence from a prospective study. Am J Epidemiol 135(5):490–499
Marsh D, Akesson K, Beaton DE, Bogoch ER, Boonen S, Brandi ML, McLellan AR, Mitchell PJ, Sale JE, Wahl DA (2011) Coordinator-based systems for secondary prevention in fragility fracture patients. Osteoporos Int 22(7):2051–2065. doi:10.1007/s00198-011-1642-x
The National guidelines for Musculoskeletal Diseases by The National Board of Health and Welfare (2012)
Bolland MJ, Grey AB, Gamble GD, Reid IR (2010) Effect of osteoporosis treatment on mortality: a meta-analysis. J Clin Endocrinol Metab 95(3):1174–1181. doi:10.1210/jc.2009-0852
Dell R, Greene D, Schelkun SR, Williams K (2008) Osteoporosis disease management: the role of the orthopaedic surgeon. J Bone Joint Surg Am 90(Suppl 4):188–194. doi:10.2106/JBJS.H.00628
Kothawala P, Badamgarav E, Ryu S, Miller RM, Halbert RJ (2007) Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin Proc 82(12):1493–1501. doi:10.1016/s0025-6196(11)61093-8
Acknowledgments
The authors gratefully acknowledge the support from the research fund at Skaraborg Hospital, Skövde, Sweden, and in particular Salmir Nasic for statistical support. We also wish to thank Hulda Vigfusdottir, Anette Carlsson and John Karlsson Sondell for extensive data collection as well as all staff involved in the FLS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Compliance with ethical standards
The study was approved by the regional ethical review board in Gothenburg.
Conflicts of interest
None.
Funding sources
This study was funded by the research fund at Skaraborg Hospital Skövde, Sweden, the Swedish Research Council (VR), the Gothenburg Society of Medicine (GLS), the ALF/LUA grant from the Sahlgrenska University Hospital and Gustaf V:s och Drottning Victorias Frimurarstiftelse.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Axelsson, K.F., Jacobsson, R., Lund, D. et al. Effectiveness of a minimal resource fracture liaison service. Osteoporos Int 27, 3165–3175 (2016). https://doi.org/10.1007/s00198-016-3643-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3643-2