Abstract
Extracorporeal membrane oxygenation (ECMO) has been used increasingly for both respiratory and cardiac failure in adult patients. Indications for ECMO use in cardiac failure include severe refractory cardiogenic shock, refractory ventricular arrhythmia, active cardiopulmonary resuscitation for cardiac arrest, and acute or decompensated right heart failure. Evidence is emerging to guide the use of this therapy for some of these indications, but there remains a need for additional evidence to guide best practices. As a result, the use of ECMO may vary widely across centers. The purpose of this document is to highlight key aspects of care delivery, with the goal of codifying the current use of this rapidly growing technology. A major challenge in this field is the need to emergently deploy ECMO for cardiac failure, often with limited time to assess the appropriateness of patients for the intervention. For this reason, we advocate for a multidisciplinary team of experts to guide institutional use of this therapy and the care of patients receiving it. Rigorous patient selection and careful attention to potential complications are key factors in optimizing patient outcomes. Seamless patient transport and clearly defined pathways for transition of care to centers capable of providing heart replacement therapies (e.g., durable ventricular assist device or heart transplantation) are essential to providing the highest level of care for those patients stabilized by ECMO but unable to be weaned from the device. Ultimately, concentration of the most complex care at high-volume centers with advanced cardiac capabilities may be a way to significantly improve the care of this patient population.
Similar content being viewed by others
Introduction
Extracorporeal membrane oxygenation (ECMO) is a high-risk, complex, and resource-intensive therapy. Its use for adult patients with cardiac failure and cardiac arrest has grown rapidly over the last decade in the setting of advances in extracorporeal technology [1, 2]. ECMO plays a pivotal role in providing life-saving mechanical circulatory support in selected patients. However, rigorous data supporting its use are limited [3], ECMO practices vary widely across hospitals and countries [4], and there may be room to improve outcomes in certain populations [5].
ECMO for cardiac failure should be implemented with a clear understanding of its indications, limitations, and risks, and be performed at centers that are prepared to both initiate and subsequently manage these patients, whether it be internally or by partnering closely with institutions capable of such management. There are numerous current potential uses of ECMO for cardiac failure, and additional evidence as well as standardized practices are needed to better define the optimal patient populations for this invasive and expensive therapy [6].
Purpose of this position paper
This position paper represents the expert opinion of an international group of physicians, ECMO specialists, and allied healthcare workers, spanning six continents, who have expertise relevant to mechanical circulatory support (MCS) used to treat patients with severe cardiac failure. Many practices in the care of patients with cardiogenic shock are not supported by randomized, controlled data and for this reason we seek to offer expert opinion to provide some guidance for the use of ECMO as circulatory support. A companion position paper was previously published addressing organizational issues for centers utilizing ECMO for acute respiratory failure [7]. The aim of this paper is to provide clinicians, ECMO center directors and coordinators, hospital administrators, healthcare organizations, and regional, national, and international policy makers a consensus approach to the organization of ECMO programs for cardiac failure and cardiac arrest in adults. Our goal is that this will help ensure ECMO is delivered appropriately, safely, and proficiently. Given that ECMO is being widely used across centers of varying case volume and experience, a key message of this paper is that ECMO should be performed within a framework of cooperating hospitals (Table 1). Centers with limited resources should have collaborative relationships with high-volume centers capable of providing long-term cardiac support therapies, such as durable ventricular assist devices (VAD) and heart transplantation.
ECMO in the spectrum of mechanical circulatory support devices
Venoarterial ECMO is one of several short-term, or temporary, MCS options, i.e., catheter- or cannula-based vascular access with mechanical pumps that are used as first-line rescue therapy in patients with refractory cardiogenic shock [8, 9]. Other short-term MCS devices used in this setting include intra-aortic balloon pumps (IABP), percutaneous VADs, and surgical VADs [10,11,12]. The differences between the various short-term MCS devices are outlined in Table 2 [10]. The use of different MCS devices is not necessarily exclusive, as IABP or percutaneous VADs, for instance, may be used to manage left ventricular over-distention in patients receiving venoarterial ECMO [13]. Similarly, ECMO may be initiated for a patient inadequately supported by a percutaneous VAD. It is important to note that, although the terminology we use throughout the paper is in common usage in the field, there is currently no universally accepted nomenclature for mechanical circulatory support devices.
Indications and contraindications for venoarterial ECMO
There are several etiologies of cardiac failure that are supportable with ECMO. These can be broadly classified into severe, refractory forms of cardiogenic shock, i.e., cardiogenic shock despite the use of multiple vasoactive medications or an MCS device (i.e., Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) level 1); cardiac arrest; refractory ventricular arrhythmias; and acute or decompensated right heart failure in the context of pulmonary vascular disease (e.g., pulmonary hypertension or pulmonary embolism) [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. Table 3 provides a list of indications (with the corresponding level of evidence) and contraindications to venoarterial ECMO [32, 33].
Proposed nomenclature for cardiac centers
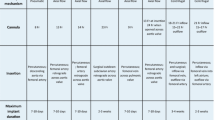
Access to and experience with ECMO and other MCS devices, inter-hospital ECMO transport, long-term heart replacement therapies, and other cardiac procedures help inform the appropriate strategy for ECMO use. Among centers with potential access to ECMO, we define local centers as those that may or may not have interventional cardiology facilities available on-site. Referral centers are centers with access to cardiac catheterization and percutaneous left ventricular assist devices. Regional referral centers have access to short-term MCS devices with the ability to perform inter-hospital transport on MCS. Comprehensive care centers have all of the resources of regional referral centers with the additional capability of performing long-term MCS and heart transplantation. Proposed relationships between centers are shown in Fig. 1.
Referral model for ECMO for cardiac failure in adults. Local center: may or may not have interventional cardiology facilities available on-site. Referral center: access to cardiac catheterization and percutaneous LV assistance devices ± ECMO. Regional referral center: access to short-term MCS devices including ECMO with ability to perform inter-hospital transport on MCS. Comprehensive care center: regional referral center + long-term MCS and heart transplantation
Organization of ECMO centers for cardiac failure
The implementation of ECMO for cardiac failure differs from that for respiratory failure because of the more frequent need for emergent ECMO support. Furthermore, some etiologies of cardiac failure require prompt intervention to reverse the underlying pathology (e.g., percutaneous coronary intervention in an acute coronary syndrome), which, in turn, may substantially shorten the duration of circulatory support necessary for survival. For both of these reasons, the decision to initiate ECMO or other MCS must be made expeditiously. ECMO for cardiac failure would ideally be performed at regional referral or comprehensive care centers, where ECMO is part of a broader management strategy for advanced cardiovascular disease that may incorporate percutaneous coronary interventions, long-term MCS, and heart transplantation [32]. Such centers should have algorithms in place to assist with rapid decision-making, and ideally be able to quickly convene a multidisciplinary “heart team” or “shock team,” which may consist of cardiothoracic surgeons, interventional cardiologists, heart failure specialists, critical care physicians, and any other team members deemed essential who can pursue the most appropriate management strategy [34,35,36,37]. However, this does not preclude the use of ECMO at less experienced or less well-resourced centers, especially in cooperation with a more experienced center.
The use of ECMO to restore circulation during cardiac arrest, referred to as extracorporeal cardiopulmonary resuscitation (ECPR), presents its own unique set of challenges. Unlike progressive, severe cardiogenic shock, which most often will occur in the cardiac catheterization laboratory, intensive care unit, or operating room, cardiac arrest may occur unpredictably in any location throughout the hospital, including emergency departments, where ECPR programs have been increasingly under development. ECPR may also take place in the pre-hospital setting, which is a venue under active investigation [38,39,40,41]. Although the specific needs of ECPR programs are beyond the scope of this paper, we strongly recommend that these programs be linked to inpatient intensive care unit programs that are capable of managing the cases wherever they are initiated, or that rapid transfer to a partner center for further management can be guaranteed. Regardless of the location, both the decision to initiate (or not initiate) ECMO, and the cannulation itself, must be executed as quickly as possible, ideally with the use of pre-specified criteria to identify patients most (or least) likely to benefit from ECPR (Table 4) [42, 43]. A rapid response team, similar to the aforementioned multidisciplinary team for cardiogenic shock, helps facilitate the successful implementation of ECPR [22, 23, 44, 45]. An approach utilizing smaller arterial cannulas inserted percutaneously under ultrasound guidance, or under fluoroscopic guidance in the catheterization laboratory, should be encouraged to reduce hemorrhagic, infectious, and vascular complications [46].
It should be emphasized that ECMO is a short-term means of supporting the patient’s circulation but does not treat the underlying pathology. As soon as feasible (and in some cases emergently), this underlying pathology must be aggressively managed to optimize the chance of recovery and expedite safe weaning from ECMO. This includes but is not limited to revascularization (percutaneously or surgically) for patients with ischemic heart disease as the etiology [47, 48], valvular surgery for patients with a valvular etiology, medical or ablative therapy for those with refractory arrhythmia [49], and thrombolysis (systemic or catheter-directed), pulmonary embolectomy, and pulmonary endarterectomy for patients with acute or chronic thromboembolic disease [50]. For those with little chance of ventricular recovery or safe weaning from support, early evaluation for heart replacement therapies should be initiated. In addition to having advanced cardiac therapies available, centers performing ECMO to support patients with severe pulmonary vascular disease should have access to clinicians with expertise in pulmonary hypertension management and, ideally, the ability to perform lung transplantation [28].
Higher ECMO case volume at a given hospital has been associated with lower in-hospital mortality [51], suggesting that referral to high-volume ECMO centers may lead to improved outcomes [52,53,54]. Cannulation may be performed by either the originating hospital or the receiving hospital at the time of transport, depending on the capabilities of each center and the agreement between them [55]. Centers initiating ECMO should have surgical services immediately available that can manage the potentially life- or limb-threatening vascular complications of cannulation.
For local and referral centers without the ability to initiate ECMO, we recommend the creation of networks around regional referral or comprehensive care centers capable of deploying mobile ECMO teams to initiate this therapy and transfer these patients. If ECMO is initiated by local or referral centers for resuscitation of a patient with severe cardiogenic shock or cardiac arrest, including as back-up to high-risk cardiac interventions, the patient may be exposed to substantial risk and suboptimal outcomes. For these centers, we recommend a formal partnership with regional referral or comprehensive care centers willing to accept these patients for transfer (with mutual agreement of indications, contraindications, criteria for initiation, and technique for cannulation) [56]. These approaches have been successfully adopted for respiratory ECMO centers with favorable results [53, 57, 58].
The minimum acceptable case volume for an ECMO center remains controversial. Recent data from an analysis of 290 centers within the Extracorporeal Life Support Organization (ELSO) registry suggest an inverse linear relationship between case volume and mortality, with centers performing more than 30 adult ECMO cases per year (from 2008 to 2013) having a significantly lower mortality than centers performing fewer than 6 cases per year (adjusted OR 0.61, 95% CI 0.46–0.80), a relationship that held true when the analysis was limited to ECMO for cardiac failure [51]. However, it is important to note that this threshold was determined on the basis of retrospective registry data from centers whose levels of expertise were not specified.
As with any therapy, familiarity with ECMO will improve outcomes as the potential complications can be recognized and addressed quickly at centers where volumes are high. Therefore, to optimize outcomes for this therapy we recommend that a reasonable goal should be a minimum case volume of 30 adult ECMO cases per year, with a substantial proportion being for cardiac failure [51]. This case volume goal should be achieved through evidence-based practices and protocols where available, and otherwise through expert consensus. However, centers should resist the temptation to perform ECMO only to increase center volume as a way of meeting this or any other volume goal. Adult cardiac ECMO centers should work in close coordination with their pediatric and adult respiratory ECMO colleagues. In particular, because some patients with severe refractory cardiac failure may develop concomitant severe respiratory failure, cardiac ECMO programs should have access to clinicians or to a center with expertise in the use of ECMO for respiratory failure, ideally within their own center. Clinical competence should be developed and maintained through local, national, and international educational programs with an emphasis on both user-specific and multidisciplinary aspects of such training. Multiple medical societies and other organizations, including ELSO, offer training and education in the provision of ECMO.
Patient selection criteria
To guide decision-making and optimize outcomes, standardized pre-specified ECMO criteria should be created whenever possible. These criteria may differ from one disease process to another, and often depend on the anticipated duration of support and likelihood of weaning from mechanical circulatory support. For some indications, there are not enough data to create definitive criteria. This is particularly important for programs performing both ECPR as well as ECMO for cardiogenic shock, where decisions often have to be made with limited clinical information. For centers initiating ECMO with the intention of referral to a tertiary care center, pre-established inclusion and exclusion criteria as well as the approach to cannulation should be agreed on between referring and receiving hospitals to ensure acceptance of the patient by the receiving hospital. Standardization of equipment, where feasible, is also advised. The recent development of prognostic scoring systems, such as the Survival After Venoarterial ECMO (SAVE) score, may help guide clinicians in selecting appropriate candidates for ECMO [47, 59]. More research is needed to help establish disease-specific criteria to provide the best estimation of favorable clinical outcomes and to externally validate predictive survival models.
Mobile ECMO teams
High-volume ECMO centers, particularly those serving as the regional referral or comprehensive care centers within hospital referral networks, should ideally establish and coordinate mobile ECMO teams to retrieve patients with severe cardiac failure refractory to conventional therapy. These mobile teams should be available 24 h a day, 7 days a week, and employ experienced personnel trained in transporting critically ill patients, insertion of cannulae (if performed by the mobile team), as well as circuit and patient management. The team should include some combination of physicians, surgeons, transport specialists, nurses, perfusionists, or other ECMO specialists. Imaging requirements at the referring hospital should be considered, including echocardiography or fluoroscopy. Portable ultrasound equipment is essential to aid in vascular access. Checklists should be considered to ensure availability of all necessary equipment and consistency of provider roles and actions before and during transport. After-action reviews are recommended. Successful transportation of patients on cardiopulmonary support by ambulance, helicopter, and fixed-wing aircraft has been described [60,61,62]. Centers performing ECMO should develop specific guidelines and ensure adequate staff training to provide uninterrupted availability to intrahospital transport of patients receiving ECMO. The equipment used for transport should meet the relevant standards for ground or air transport, with an emphasis on safety and durability.
Recommendations for physical facilities and equipment
The equipment that should be readily available is listed in Table 5. Importantly, a primed circuit should be available at all times in case of emergency, which should be possible in centers with sufficient ECMO volume. Some evidence suggests that primed circuits may be able to be stored for up to 4 weeks without increased risk of infection [63, 64]. Availability of both staff and equipment should allow for rapid circuit exchange in case of sudden circuit malfunction. For programs performing ECPR, the same staff and equipment availability should allow for the possibility of initiating cannulation within 15 min of conventional CPR onset in any location within the hospital (Table 4). Rapid initiation of extracorporeal support may require pre-primed circuits and other essential equipment to be stored in multiple locations throughout the facility. An organized approach to ECPR should be undertaken with a clearly delineated team, ideally available 24 h per day, that can respond immediately to in-hospital cardiac arrests.
Staffing
All staff involved in ECMO should meet the requirements of their subspecialty training as set forth by their specific governing body [65]. The director of the cardiac ECMO program should be a board-certified cardiovascular specialist with expertise in critical care; a thoracic, vascular, or trauma surgeon; or other board-certified specialist with specific training and experience in ECMO. Every member of the team should receive specific ECMO training and demonstrate competencies on an ongoing basis. There should be 24-h availability of an on-call physician comfortable with managing patients receiving ECMO both to assist with urgent or emergent management of patients and to evaluate patients from referring hospitals. Selected members of the ECMO team should be trained in vascular and cardiac ultrasonography for insertion, maintenance, and surveillance of the ECMO device. Fully trained ECMO specialists should be immediately available for circuit-related concerns, including ECMO circuit exchange.
ECMO specialists should be trained to prime and set up the circuit. Depending on the center-specific caregiver model, the ECMO specialist may also be responsible for managing equipment and supplies, daily rounds, troubleshooting, education, and performing administrative duties. An ECMO coordinator (often one of the lead ECMO specialists) is essential to assist the medical director with various aspects of the ECMO program, including but not limited to training, staffing, quality improvement, and patient data entry into the ELSO registry or other relevant databases.
ECMO staff should receive regular training and education on theoretical and practical aspects of ECMO, including simulation training whenever available [66]. Participation of staff in this education program should be recorded and their proficiency evaluated, with retraining of team members as needed, on the basis of criteria set forth by the ECMO program [65]. Standardization of assessing proficiency should be a goal for the ECMO community. Roles and responsibilities for staff who manage specific aspects of patient care, including circuit setting adjustments, ventilator changes, anticoagulation, and cannula care and adjustments, should be clearly outlined, with role-specific training organized by the ECMO program. Training and education materials as well as practical courses in ECMO are available through ELSO and other major medical organizations [67]. For institutions starting new ECMO programs, adequate planning and training by qualified personnel is necessary prior to performing ECMO. Consultation from experienced personnel at other centers is advisable.
Non-ECMO services
Various personnel from medical, surgical, and laboratory services should be available 24 h per day to assist with management of patients receiving ECMO support (Table 6). Ideally services needed for ECPR would be available in-house at all times. An ECMO center should be able to provide cardiothoracic surgery, percutaneous coronary intervention, vascular and abdominal surgery, and interventional radiology services on an emergent basis. The hospital’s biomedical engineering department should maintain ECMO equipment on a regular basis. Physical and occupational therapy should be available on a non-urgent basis, with specific training in optimizing patient mobility on ECMO [68]. Pastoral and palliative care, along with other patient and family support services, should be available, at least on a non-urgent basis, in regions where such services exist. Pre-emptive palliative care consultation prior to ECMO initiation may be appropriate for patients in whom outcomes are especially uncertain, such as those in patients where ECMO is used as a “bridge to decision” [69]. Access to ethics consultation, similarly, may be of particular importance for situations in which patients are dependent on ECMO for survival but without an option for destination therapy, a so-called bridge to nowhere scenario [70, 71]. In order to help prepare patients’ families and manage expectations, access to written materials about ECMO may be useful.
Program evaluation and quality assurance
The ECMO program should have procedures in place to perform quality assurance for internal ECMO program evaluation [65, 67]. Each ECMO center should hold routine multidisciplinary meetings to analyze its activity and review its equipment needs. Regularly scheduled meetings should be organized among ECMO centers and other referring hospitals within a given ECMO network to report activities and review cases. Any major complication or death should undergo prompt review by the appropriate ECMO team members and the hospital committee responsible for oversight of such adverse outcomes, adherent to the relevant quality assurance laws. Formal clinical-pathological case reviews with a multidisciplinary approach should also be conducted regularly.
Documentation of the maintenance of equipment and supplies should be performed. Data reports summarizing the indications for and results of ECMO should be available for quality assurance review. ECMO centers are strongly encouraged to submit their data to large national or international databases for clinical audit and benchmarking, to allow for comparison of outcomes and to highlight variation with other national and international institutions. Regional and national accreditation organizations should be created to establish guidelines for best practices, thereby allowing ECMO programs to be evaluated regularly for adherence to accepted standards. Centers with poorer than expected results should be encouraged to engage in extensive practice evaluation and improvement strategies. This review should be constructed to identify strengths and weaknesses within the program to help ensure its sustainability. We recommend that new programs create an advisory committee consisting of experts from outside the institution to assist with program development and quality review, which could allow for an appropriate period of oversight for new programs to ensure the new center is meeting acceptable standards. Cost considerations vary across centers and countries, and should be evaluated on the basis of local needs.
Patient follow-up
Each ECMO center should ensure appropriate short- and long-term follow-up for patients who survive having received ECMO, with specialty-specific consultation as needed, particularly for those with ongoing heart failure and those at risk for delayed mortality. Physical rehabilitation programs may be of particular importance given the potential for ICU-acquired weakness in patients requiring prolonged MCS. Psychological and cognitive rehabilitation may also be important with survivors, as well as screening for psychological distress post-discharge.
Research
There is an ongoing need for controlled clinical trials and other high-level evidence to clarify the appropriate use of ECMO in severe refractory cardiogenic shock and other cardiac indications. These data will help to guide clinicians with respect to disease-specific indications and contraindications. Given the relatively small number of ECMO cases at any individual center, and the large numbers of patients needed to study meaningful clinical outcomes, national and international organizations of ECMO centers are useful; two such organizations are ELSO (https://www.elso.org) and the International ECMO Network (ECMONet, https://www.internationalecmonetwork.org). ELSO maintains the largest registry of ECMO patients, which has proven to be a valuable tool for researchers. Similarly, ECMONet is a research consortium that includes ECMO-specific expertise dedicated to conducting and supporting high-quality, high-impact research in the field. Both randomized controlled trials and matched pairs trials may be suitable for studying these patients with severe cardiac failure, several of which are either being conducted or in the planning phases [41, 53, 72–76].
Conclusions
ECMO for cardiac failure is a high-risk and complex therapy. ECMO will very likely continue to play a vital role in the management of cardiovascular failure, and it should be performed responsibly within a given center or within a network of centers, by clinicians with the appropriate expertise. More precisely defining the role of ECMO in cardiac failure, and the optimal techniques that should be used, will require further evidence. Ongoing technological developments and future research will no doubt spur continued evolution in the field.
References
Paden ML, Rycus PT, Thiagarajan RR, Registry E (2014) Update and outcomes in extracorporeal life support. Semin Perinatol 38:65–70
Stretch R, Sauer CM, Yuh DD, Bonde P (2014) National trends in the utilization of short-term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol 64:1407–1415
Combes A, Brodie D, Chen YS, Fan E, Henriques JPS, Hodgson C, Lepper PM, Leprince P, Maekawa K, Muller T, Nuding S, Ouweneel DM, Roch A, Schmidt M, Takayama H, Vuylsteke A, Werdan K, Papazian L (2017) The ICM research agenda on extracorporeal life support. Intensive Care Med 43:1306–1318
Abrams D, Combes A, Brodie D (2014) What’s new in extracorporeal membrane oxygenation for cardiac failure and cardiac arrest in adults? Intensive Care Med 40:609–612
Karagiannidis C, Brodie D, Strassmann S, Stoelben E, Philipp A, Bein T, Muller T, Windisch W (2016) Extracorporeal membrane oxygenation: evolving epidemiology and mortality. Intensive Care Med 42:889–896
Werdan K, Gielen S, Ebelt H, Hochman JS (2014) Mechanical circulatory support in cardiogenic shock. Eur Heart J 35:156–167
Combes A, Brodie D, Bartlett R, Brochard L, Brower R, Conrad S, De Backer D, Fan E, Ferguson N, Fortenberry J, Fraser J, Gattinoni L, Lynch W, MacLaren G, Mercat A, Mueller T, Ogino M, Peek G, Pellegrino V, Pesenti A, Ranieri M, Slutsky A, Vuylsteke A, International ECMO Network (ECMONet) (2014) Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med 190:488–496
Abrams D, Combes A, Brodie D (2014) Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol 63:2769–2778
van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, Thiele H, Washam JB, Cohen MG, American Heart Association Council on Clinical Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Quality of Care and Outcomes Research, Mission: Lifeline (2017) Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation 136:e232–e268
Garan AR, Rabbani LE (2015) Coronary artery interventions in cardiogenic shock. In: Lanzer P (ed) PanVascular medicine. Springer, Berlin, pp 2173–2203
Sayer GT, Baker JN, Parks KA (2012) Heart rescue: the role of mechanical circulatory support in the management of severe refractory cardiogenic shock. Curr Opin Crit Care 18:409–416
Khera R, Cram P, Lu X, Vyas A, Gerke A, Rosenthal GE, Horwitz PA, Girotra S (2015) Trends in the use of percutaneous ventricular assist devices analysis of national inpatient sample data, 2007 through 2012. JAMA Intern Med 175:941–950
Truby LK, Takeda K, Mauro C, Yuzefpolskaya M, Garan AR, Kirtane AJ, Topkara VK, Abrams D, Brodie D, Colombo PC, Naka Y, Takayama H (2017) Incidence and Implications of left ventricular distention during venoarterial extracorporeal membrane oxygenation support. ASAIO J 63:257–265
Stevenson LW, Pagani FD, Young JB, Jessup M, Miller L, Kormos RL, Naftel DC, Ulisney K, Desvigne-Nickens P, Kirklin JK (2009) INTERMACS profiles of advanced heart failure: the current picture. J Heart Lung Transpl 28:535–541
Reynolds HR, Hochman JS (2008) Cardiogenic shock: current concepts and improving outcomes. Circulation 117:686–697
Ostadal P, Rokyta R, Kruger A, Vondrakova D, Janotka M, Smid O, Smalcova J, Hromadka M, Linhart A, Belohlavek J (2017) Extra corporeal membrane oxygenation in the therapy of cardiogenic shock (ECMO-CS): rationale and design of the multicenter randomized trial. Eur J Heart Fail 19(Suppl 2):124–127
Sakamoto S, Taniguchi N, Nakajima S, Takahashi A (2012) Extracorporeal life support for cardiogenic shock or cardiac arrest due to acute coronary syndrome. Ann Thorac Surg 94:1–7
Rastan AJ, Dege A, Mohr M, Doll N, Falk V, Walther T, Mohr FW (2010) Early and late outcomes of 517 consecutive adult patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. J Thorac Cardiovasc Surg 139:302–311
Fayssoil A, Nardi O, Orlikowski D, Combes A, Chastre J, Annane D (2010) Percutaneous extracorporeal membrane oxygenation for cardiogenic shock due to acute fulminant myocarditis. Ann Thorac Surg 89:614–616
Thiele H, Ohman EM, Desch S, Eitel I, de Waha S (2015) Management of cardiogenic shock. Eur Heart J 36:1223–1230
Truby L, Mundy L, Kalesan B, Kirtane A, Colombo PC, Takeda K, Fukuhara S, Naka Y, Takayama H (2015) Contemporary outcomes of venoarterial extracorporeal membrane oxygenation for refractory cardiogenic shock at a large tertiary care center. ASAIO J 61:403–409
Chen YS, Lin JW, Yu HY, Ko WJ, Jerng JS, Chang WT, Chen WJ, Huang SC, Chi NH, Wang CH, Chen LC, Tsai PR, Wang SS, Hwang JJ, Lin FY (2008) Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet 372:554–561
Shin TG, Jo IJ, Sim MS, Song YB, Yang JH, Hahn JY, Choi SH, Gwon HC, Jeon ES, Sung K, Lee YT, Choi JH (2013) Two-year survival and neurological outcome of in-hospital cardiac arrest patients rescued by extracorporeal cardiopulmonary resuscitation. Int J Cardiol 168:3424–3430
Ouweneel DM, Schotborgh JV, Limpens J, Sjauw KD, Engstrom AE, Lagrand WK, Cherpanath TG, Driessen AH, de Mol BA, Henriques JP (2016) Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta-analysis. Intensive Care Med 42:1922–1934
Abrams DC, Brodie D, Rosenzweig EB, Burkart KM, Agerstrand CL, Bacchetta MD (2013) Upper-body extracorporeal membrane oxygenation as a strategy in decompensated pulmonary arterial hypertension. Pulm Circ 3:432–435
Javidfar J, Brodie D, Sonett J, Bacchetta M (2012) Venovenous extracorporeal membrane oxygenation using a single cannula in patients with pulmonary hypertension and atrial septal defects. J Thorac Cardiovasc Surg 143:982–984
Camboni D, Akay B, Sassalos P, Toomasian JM, Haft JW, Bartlett RH, Cook KE (2011) Use of venovenous extracorporeal membrane oxygenation and an atrial septostomy for pulmonary and right ventricular failure. Ann Thorac Surg 91:144–149
Rosenzweig EB, Brodie D, Abrams DC, Agerstrand CL, Bacchetta M (2014) Extracorporeal membrane oxygenation as a novel bridging strategy for acute right heart failure in group 1 pulmonary arterial hypertension. ASAIO J 60:129–133
Abrams D, Brodie D (2013) Emerging indications for extracorporeal membrane oxygenation in adults with respiratory failure. Ann Am Thorac Soc 10:371–377
Maggio P, Hemmila M, Haft J, Bartlett R (2007) Extracorporeal life support for massive pulmonary embolism. J Trauma 62:570–576
Corsi F, Lebreton G, Brechot N, Hekimian G, Nieszkowska A, Trouillet JL, Luyt CE, Leprince P, Chastre J, Combes A, Schmidt M (2017) Life-threatening massive pulmonary embolism rescued by venoarterial-extracorporeal membrane oxygenation. Crit Care 21:76
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Filippatos G, McMurray JJV, Aboyans V, Achenbach S, Agewall S, Al-Attar N, Atherton JJ, Bauersachs J, Camm AJ, Carerj S, Ceconi C, Coca A, Elliott P, Erol C, Ezekowitz J, Fernandez-Golfin C, Fitzsimons D, Guazzi M (2016) 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 18:891–975
Rihal CS, Naidu SS, Givertz MM, Szeto WY, Burke JA, Kapur NK, Kern M, Garratt KN, Goldstein JA, Dimas V, Tu T, Society for Cardiovascular Angiography and Interventions (SCAI), Heart Failure Society of America (HFSA), Society of Thoracic Surgeons (STS), American Heart Association (AHA), and American College of Cardiology (ACC) (2015) 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care endorsed by the American Heart Association, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista; affirmation of value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention. J Am Coll Cardiol 65:2140–2141
Garan AR, Kirtane A, Takayama H (2016) Redesigning care for patients with acute myocardial infarction complicated by cardiogenic shock: the “shock team”. JAMA Surg 151:684–685
Tchantchaleishvili V, Hallinan W, Massey HT (2015) Call for organized statewide networks for management of acute myocardial infarction-related cardiogenic shock. JAMA Surg 150:1025–1026
Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Uva MS, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos N, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Pepper J, Anyanwum A, Badimon L, Bauersachs J, Baumbach A, Beygui F, Bonaros N, De Carlo M, Dobrev D, Dunning J, Eeckhout E, Gielen S, Luckraz H, Mahrholdt H, Montalescot G, Paparella D, Rastan AJ, Sanmartin M, Sergeant P, Silber S, Tamargo J, ten Berg J, Thiele H, van Geuns RJ, Wagner HO, Wassmann S, Wendler O, Weidinger F, Ibrahimov F, Legrand V, Terzic I, Postadzhiyan A, Skoric B, Georgiou GM, Zelizko M, Junker A, Eha J, Romppanen H, Bonnet JL, Aladashvili A, Hambrecht R, Becker D, Gudnason T, Segev A, Bugiardini R, Sakhov O, Mirrakhimov A, Pereira B, Felice H, Trovik T, Dudek D, Pereira H, Nedeljkovic MA, Hudec M, Cequier A, Erlinge D, Roffi M, Kedev S, Addad F, Yildirir A, Davies J, Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) (2014) 2014 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 35:U2152–U2541
Atkinson TM, Ohman EM, O’Neill WW, Rab T, Cigarroa JE, Amer ISC (2016) A practical approach to mechanical circulatory support in patients undergoing percutaneous coronary intervention an interventional perspective. JACC Cardiovasc Interv 9:871–883
Tonna JE, Johnson NJ, Greenwood J, Gaieski DF, Shinar Z, Bellezo JM, Becker L, Shah AP, Youngquist ST, Mallin MP, Fair JF 3rd, Gunnerson KJ, Weng C, McKellar S, Extracorporeal REsuscitation ConsorTium (ERECT) Research Group (2016) Practice characteristics of emergency department extracorporeal cardiopulmonary resuscitation (eCPR) programs in the United States: the current state of the art of emergency department extracorporeal membrane oxygenation (ED ECMO). Resuscitation 107:38–46
Lamhaut L, Jouffroy R, Soldan M, Phillipe P, Deluze T, Jaffry M, Dagron C, Vivien B, Spaulding C, An K, Carli P (2013) Safety and feasibility of prehospital extra corporeal life support implementation by non-surgeons for out-of-hospital refractory cardiac arrest. Resuscitation 84:1525–1529
Lamhaut L, Hutin A, Puymirat E, Jouan J, Raphalen JH, Jouffroy R, Jaffry M, Dagron C, An K, Dumas F, Marijon E, Bougouin W, Tourtier JP, Baud F, Jouven X, Danchin N, Spaulding C, Carli P (2017) A pre-hospital extracorporeal cardio pulmonary resuscitation (ECPR) strategy for treatment of refractory out hospital cardiac arrest: an observational study and propensity analysis. Resuscitation 117:109–117
Assistance Publique - Hôpitaux de Paris (2000) A comparative study between a pre-hospital and an in-hospital circulatory support strategy (ECMO) in refractory cardiac arrest (ACPAR2). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). [cited 2017 Jun 27]. https://clinicaltrials.gov/ct2/show/NCT02527031. NLM Identifier: NCT02527031
Callaway CW, Soar J, Aibiki M, Bottiger BW, Brooks SC, Deakin CD, Donnino MW, Drajer S, Kloeck W, Morley PT, Morrison LJ, Neumar RW, Nicholson TC, Nolan JP, Okada K, O’Neil BJ, Paiva EF, Parr MJ, Wang TL, Witt J, Advanced Life Support Chapter Collaborators (2015) Part 4: advanced life support 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation 132:S84–S145
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P (2016) 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 37:U2129–U2130
Shin TG, Choi JH, Jo IJ, Sim MS, Song HG, Jeong YK, Song YB, Hahn JY, Choi SH, Gwon HC, Jeon ES, Sung K, Kim WS, Lee YT (2011) Extracorporeal cardiopulmonary resuscitation in patients with inhospital cardiac arrest: a comparison with conventional cardiopulmonary resuscitation. Crit Care Med 39:1–7
Maekawa K, Tanno K, Hase M, Mori K, Asai Y (2013) Extracorporeal cardiopulmonary resuscitation for patients with out-of-hospital cardiac arrest of cardiac origin: a propensity-matched study and predictor analysis. Crit Care Med 41:1186–1196
Takayama H, Landes E, Truby L, Fujita K, Kirtane AJ, Mongero L, Yuzefpolskaya M, Colombo PC, Jorde UP, Kurlansky PA, Takeda K, Naka Y (2015) Feasibility of smaller arterial cannulas in venoarterial extracorporeal membrane oxygenation. J Thorac Cardiov Surg 149:1428–1433
Muller G, Flecher E, Lebreton G, Luyt CE, Trouillet JL, Brechot N, Schmidt M, Mastroianni C, Chastre J, Leprince P, Anselmi A, Combes A (2016) The ENCOURAGE mortality risk score and analysis of long-term outcomes after VA-ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Med 42:370–378
Garan AR, Eckhardt C, Takeda K, Topkara VK, Clerkin K, Fried J, Masoumi A, Demmer RT, Trinh P, Yuzefpolsakaya M, Naka Y, Burkhoff D, Kirtane A, Colombo PC, Takayama H (2017) Predictors of survival and ability to wean from short-term mechanical circulatory support device following acute myocardial infarction complicated by cardiogenic shock. Eur Heart J Acute Cardiovasc Care. https://doi.org/10.1177/2048872617740834
Baratto F, Pappalardo F, Oloriz T, Bisceglia C, Vergara P, Silberbauer J, Albanese N, Cireddu M, D’Angelo G, Di Prima AL, Monaco F, Paglino G, Radinovic A, Regazzoli D, Silvetti S, Trevisi N, Zangrillo A, Della Bella P (2016) Extracorporeal membrane oxygenation for hemodynamic support of ventricular tachycardia ablation. Circ Arrhythm Electrophysiol 9:e004492
Dudzinski DM, Giri J, Rosenfield K (2017) Interventional treatment of pulmonary embolism. Circ Cardiovasc Interv 10:e004345
Barbaro RP, Odetola FO, Kidwell KM, Paden ML, Bartlett RH, Davis MM, Annich GM (2015) Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med 191:894–901
Broman LM, Holzgraefe B, Palmer K, Frenckner B (2015) The Stockholm experience: interhospital transports on extracorporeal membrane oxygenation. Crit Care 19:278
Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, Sadique MZ, Sekhon JS, McAuley DF, Firmin RK, Harvey C, Cordingley JJ, Price S, Vuylsteke A, Jenkins DP, Noble DW, Bloomfield R, Walsh TS, Perkins GD, Menon D, Taylor BL, Rowan KM (2011) Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 306:1659–1668
Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D (2009) Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 374:1351–1363
Jaroszewski DE, Kleisli T, Staley L, Pierce C, Scott R, Steidley DE, DeValeria P, Arabia FA (2011) A traveling team concept to expedite the transfer and management of unstable patients in cardiopulmonary shock. J Heart Lung Transpl 30:618–623
Gonzalez-Stawinski GV, Chang AS, Navia JL, Banbury MK, Buda T, Hoercher K, Starling RC, Taylor DO, Smedira NG (2006) Regional referral system for patients with acute mechanical support: experience at the Cleveland Clinic Foundation. ASAIO J 52:445–449
Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, Forrest P, Gattas D, Granger E, Herkes R, Jackson A, McGuinness S, Nair P, Pellegrino V, Pettila V, Plunkett B, Pye R, Torzillo P, Webb S, Wilson M, Ziegenfuss M (2009) Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA 302:1888–1895
Patroniti N, Zangrillo A, Pappalardo F, Peris A, Cianchi G, Braschi A, Iotti GA, Arcadipane A, Panarello G, Ranieri VM, Terragni P, Antonelli M, Gattinoni L, Oleari F, Pesenti A (2011) The Italian ECMO network experience during the 2009 influenza A(H1N1) pandemic: preparation for severe respiratory emergency outbreaks. Intensive Care Med 37:1447–1457
Schmidt M, Burrell A, Roberts L, Bailey M, Sheldrake J, Rycus PT, Hodgson C, Scheinkestel C, Cooper DJ, Thiagarajan RR, Brodie D, Pellegrino V, Pilcher D (2015) Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J 36:2246–2256
Biscotti M, Agerstrand C, Abrams D, Ginsburg M, Sonett J, Mongero L, Takayama H, Brodie D, Bacchetta M (2015) One hundred transports on extracorporeal support to an extracorporeal membrane oxygenation center. Ann Thorac Surg 100:34–40
Bryner B, Cooley E, Copenhaver W, Brierley K, Teman N, Landis D, Rycus P, Hemmila M, Napolitano LM, Haft J, Park PK, Bartlett RH (2014) Two decades’ experience with interfacility transport on extracorporeal membrane oxygenation. Ann Thorac Surg 98:1363–1370
Beurtheret S, Mordant P, Paoletti X, Marijon E, Celermajer DS, Leger P, Pavie A, Combes A, Leprince P (2013) Emergency circulatory support in refractory cardiogenic shock patients in remote institutions: a pilot study (the cardiac-RESCUE program). Eur Heart J 34:112–120
Weinberg A, Miko B, Beck J, Bacchetta M, Mongero L (2015) Is it safe to leave an ECMO circuit primed? Perfusion 30:47–49
Walczak R, Lawson DS, Kaemmer D, McRobb C, McDermott P, Smigla G, Shearer I, Lodge A, Jaggers J (2005) Evaluation of a preprimed microporous hollow-fiber membrane for rapid response neonatal extracorporeal membrane oxygenation. Perfusion 20:269–275
ELSO (2013) ELSO guidelines for cardiopulmonary extracorporeal life support. Extracorporeal Life Support Organization, version 1.3 November 2013. Ann Arbor. https://www.elso.org. Accessed 29 July 2017
Zakhary BM, Kam LM, Kaufman BS, Felner KJ (2017) The utility of high-fidelity simulation for training critical care fellows in the management of extracorporeal membrane oxygenation emergencies: a randomized controlled trial. Crit Care Med 45:1367–1373
Guerguerian AM, Ogino MT, Dalton HJ, Shekerdemian LS (2013) Setup and maintenance of extracorporeal life support programs. Pediatr Crit Care Med 14:S84–S93
Abrams D, Javidfar J, Farrand E, Mongero LB, Agerstrand CL, Ryan P, Zemmel D, Galuskin K, Morrone TM, Boerem P, Bacchetta M, Brodie D (2014) Early mobilization of patients receiving extracorporeal membrane oxygenation: a retrospective cohort study. Crit Care 18:R38
Doorenbos AZ, Starks H, Bourget E, McMullan DM, Lewis-Newby M, Rue TC, Lindhorst T, Aisenberg E, Oman N, Curtis JR, Hays R, Seattle Ethics in ECLS (SEE) Consortium, Clark JD, Baden HP, Brogan TV, Di Gennaro JL, Mazor R, Roberts JS, Turnbull J, Wilfond BS (2013) Examining palliative care team involvement in automatic consultations for children on extracorporeal life support in the pediatric intensive care unit. J Palliat Med 16:492–495
Abrams DC, Prager K, Blinderman CD, Burkart KM, Brodie D (2014) Ethical dilemmas encountered with the use of extracorporeal membrane oxygenation in adults. Chest 145:876–882
Ramanathan K, Cove ME, Caleb MG, Teoh KL, Maclaren G (2015) Ethical dilemmas of adult ECMO: emerging conceptual challenges. J Cardiothorac Vasc Anesth 29:229–233
Medical University of Vienna (2000) Emergency cardiopulmonary bypass for cardiac arrest (ECPB4OHCA). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). [cited 2017 Jun 27]. https://clinicaltrials.gov/ct2/show/NCT01605409. NLM Identifier: NCT01605409
Na Homolce Hospital (2000) ExtraCorporeal Membrane Oxygenation in the Therapy of Cardiogenic Shock (ECMO-CS). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). [cited 2017 Jun 27]. https://clinicaltrials.gov/ct2/show/NCT02301819. NLM Identifier: NCT02301819
Maastricht University Medical Center (2000) Early Initiation of Extracorporeal Life Support in Refractory OHCA (INCEPTION). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). [cited 2017 Jun 27]. https://clinicaltrials.gov/ct2/show/NCT03101787. NLM Identifier: NCT03101787
University of Michigan (2000) ECPR for Refractory Out-Of-Hospital Cardiac Arrest (EROCA). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). [cited 2017 Jun 27]. https://clinicaltrials.gov/ct2/show/NCT03065647. NLM Identifier: NCT03065647
Charles University, Czech Republic (2000) Hyperinvasive Approach in Cardiac Arrest. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). [cited 2017 Jun 27]. http://clinicaltrials.gov/ct2/show/NCT01511666. NLM Identifier: NCT01511666
Funding
CH is supported by a Future Leader Fellowship from the National Heart Foundation of Australia (Award ID: 101168); funding source had no role in the writing of the manuscript or the decision to submit for publication. PKP receives funding support from the National Heart, Lung, and Blood Institute (NHLBI), National Institute of Allergy and Infectious Diseases (NIAID), Food and Drug Administration/Biomedical Advanced Research and Development Authority (FDA/BARDA), Bristol-Myers Squibb, and AtoxBio; funding sources had no role in the writing of the manuscript or the decision to submit for publication.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
DB is currently on the medical advisory boards of ALung Technologies and Kadence. All compensation for these activities is paid to Columbia University. All other authors have no conflicts of interest to report.
Rights and permissions
About this article
Cite this article
Abrams, D., Garan, A.R., Abdelbary, A. et al. Position paper for the organization of ECMO programs for cardiac failure in adults. Intensive Care Med 44, 717–729 (2018). https://doi.org/10.1007/s00134-018-5064-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-018-5064-5