Abstract
Purpose
To compare two protocols for sedation and analgesia during therapeutic hypothermia: midazolam and fentanyl versus propofol and remifentanil. The primary outcome was the time from discontinuation of infusions to extubation or decision not to extubate (offset time). Secondary outcomes were blood pressure, heart rate, use of vasopressors and inotropic drugs, pneumonia and neurological outcome.
Methods
This was an open, randomised, controlled trial on 59 patients treated with therapeutic hypothermia (33–34 °C for 24 h) after cardiac arrest in two Norwegian university hospitals between April 2008 and May 2009. The intervention was random allocation to sedation and analgesia with propofol/remifentanil or midazolam/fentanyl.
Results
Twenty-nine patients received propofol and remifentanil, and 30 midazolam and fentanyl. Baseline characteristics were similar. Sedation and analgesia were stopped in 35 patients, and extubation was performed in 17 of these. Sedation had to be continued for 24 patients. Time to offset was significantly lower in patients given propofol and remifentanil [mean (95 % confidence intervals) 13.2 (2.3–24) vs. 36.8 (28.5–45.1) h, respectively, p < 0.001]. Patients given propofol and remifentanil needed norepinephrine infusions twice as often (23 vs. 12 patients, p = 0.003). Incidence of pneumonia and 3-month neurological outcome were similar in the two groups.
Conclusions
Time to offset was significantly shorter in patients treated with propofol and remifentanil. However, the clinical course in 40 % of patients prevented discontinuation of sedation and potential benefits from a faster recovery. The propofol and remifentanil group required norepinephrine twice as often, but both protocols were tolerated in most patients.
Similar content being viewed by others
Introduction
Therapeutic hypothermia (TH) at 33–34 °C for a period of 12–24 h improves neurological recovery and survival in cardiac arrest (CA) survivors having ventricular fibrillation as the first observed arrhythmia [1–3]. Patients treated with TH need sedation and analgesia to avoid shivering and to tolerate mechanical ventilation.
Although midazolam and fentanyl is the most frequently used combination, there is a considerable variation in protocols employed for sedation and analgesia during TH [4]. Various protocols can differ in terms of haemodynamic stability, duration of mechanical ventilation and length of stay in the intensive care unit (ICU). The choice of drugs for analgesia and sedation may therefore affect morbidity and/or mortality [4]. About 50 % of patients treated with TH have a good outcome, and early prognostication after TH is important to plan further treatment. The time to offset of sedation and analgesia is important for TH patients because it defines both the time to extubation and how early residual sedation and analgesia can be separated from potential post-anoxic brain injuries.
Critically ill patients have slower and more variable drug elimination than healthy volunteers [5]. Moreover, TH may influence drug elimination [6]. Enzymatic pathways may have different sensitivity to hypothermia [7], and altered renal excretion induced by TH may vary between drugs [6, 8]. Finally, hypothermia may also affect drug–receptor binding [9].
Despite potential differences between protocols for sedation and analgesia, and the potential pharmacological changes during TH, Chamorro et al. [4] found no studies that compared protocols for sedation and analgesia during TH. Thus, we have in a randomised, controlled trial compared the currently most common protocol, midazolam and fentanyl, with a fast-offset protocol, propofol and remifentanil, for sedation and analgesia during TH following CA.
The primary objective was to compare time to offset of sedation and analgesia. The primary endpoint was the time to either extubation or a decision that extubation was clinically undesirable. An analysis of the time to extubation was also performed. Secondary outcomes were differences between study groups for circulatory variables (blood pressure, heart rate, use of vasopressors and inotropic drugs), pneumonia and cerebral performance category (CPC).
Materials and methods
Patients 18 years or older receiving TH after CA were evaluated for inclusion in this open, prospective, randomised, controlled trial. Participants were recruited in the general ICU or the coronary care unit (CCU) at St Olavs Hospital (Trondheim, Norway), or the general ICU at Stavanger University Hospital (Stavanger, Norway). Patients were excluded in cases of pregnancy, serious deteriorating circulatory status which prohibited investigational procedures (i.e. recurrent cardiac arrests), liver or renal failure defined as Sequential Organ Failure Assessment sub-scores of 3–4, history of drug allergies or contraindications for study drugs, scheduled doses of study drugs before CA or known substance abuse of opioids or benzodiazepines [10].
From CA to inclusion, patients received fractional doses of various analgesics and sedatives as required. ICU/CCU personnel included patients, performed randomisation and started allocated treatment of patients on arrival to the ICU/CCU. Concealed computerized block randomisation stratified with respect to study centre was performed by an algorithm programmed in PHP: Hypertext Preprocessor through a Web-based interface. Participants were randomised to receive sedation and analgesia with a continuous intravenous infusion of propofol and remifentanil (PR), or midazolam and fentanyl (MF) (allocation ratio 1:1).
Study infusions were started immediately after allocation. Infusions were started according to ICU/CCU standard protocols for sedation and analgesia, and titrated to motor activity assessment scale (MAAS) 0–1 (patient unresponsive or responds only to noxious stimuli) [11]. Treatments other than analgesia and sedation were given at the discretion of the attending physician. All patients were treated with mechanical ventilation. Hypothermia was induced and maintained for 24 h by external cooling, or an intravascular cooling catheter, and/or infusion of cold saline solutions. Patients were rewarmed at 0.5 °C/h until 36 °C. Circulatory support was titrated to obtain mean arterial pressure (MAP) above 70 mmHg, and hourly urine output 0.5–1 mL/kg/h. Shivering was managed with increased sedative doses, with an additional neuromuscular blocking agent if required. Seizures were treated with increased sedative doses and/or anti-epileptic drugs. Hypotension and bradycardia due to excess sedation or analgesia was treated with reduced infusion rates, followed by fluid supplementations, vasopressors or anticholinergic drugs.
Patient characteristics and medical history including details of the CA (Utstein data) were obtained from medical records [12]. Simplified acute physiology score II (SAPS II) scores were calculated [13]. Blood gas measurements (pH, lactate, PaO2 and base excess), clinical chemistry (haemoglobin, creatinine, alanine aminotransferase (ALAT), international normalized ratio of prothrombin time (PT-INR), and albumin) and hourly core temperature (rectal, bladder or vena cava inferior) were obtained. Intra-arterial blood pressure, heart rate and use of vasoactive drugs were registered hourly during the first 48 h of study drug infusion. Total net fluid balance was converted to millilitres per hour.
When a participant fulfilled the criteria for discontinuation of sedation and analgesia, the study drug infusions were stopped without tapering. To avoid acute abstinence reactions, 5 mg morphine was administered intravenously to PR patients and repeated if necessary. Criteria for stopping sedation and analgesia were circulatory stability, core temperature above 36 °C, need for respiratory pressure support of 12 cmH2O or less, and an oxygen fraction of 0.4 or less. After discontinuation of study infusions, patients were assessed hourly (for 48 h) with MAAS and Glasgow coma scale (GCS), and evaluated with respect to extubation or decision not to extubate [14]. Extubation was performed when the participant was considered able to protect his/her airway and MAAS was at least 3 (Online Resource 1). If infusions for sedation and analgesia were needed beyond 72 h, the attending physician decided the further treatment in accordance with local protocols.
A modified simplified clinical pulmonary infection score (CPIS) was used to assess pneumonia [15]. Infiltrations on x-ray and three of five other clinical signs were considered pneumonia (Table 4). Pneumonia developing after more than 48 h of mechanical ventilation was considered ventilator-associated pneumonia (VAP). Withdrawal of care was performed by the ICU/CCU. Routines for withdrawal of care included neurophysiologic examinations and were based on the American Academy of Neurology’s guideline [16]. Neurological outcome was assessed by scoring CPC in the CA group after 3 months [17, 18]. CPC 1 or 2 was considered a good outcome.
Sample size
A study on patients receiving cardiac surgery reported a time from discontinuation of sedatives and analgesics to extubation of 5.7 ± 6.6 h for MF, and 2.2 ± 4.3 h for PR [mean ± standard deviation (SD)] [19]. Other studies have shown larger differences [20, 21]. We considered a 4-h difference in the time to extubation to be of clinical interest. Using a two-tailed test, a significance level (alpha value) of 0.05 and power (beta value) of 0.80, we calculated that 18 extubated patients were required in each group. To allow for dropouts, we decided to include 40 patients, a number that was increased to 60 after 8 months of inclusion because of fewer extubations than expected.
Statistics
Data are presented as mean ± SD, median [semi-interquartile range (s-iqr)] or number of observations as appropriate. For comparison of the primary endpoint, two analyses were performed. Time to offset of sedation and analgesia, defined as the time to either extubation or to when extubation was decided to be clinically undesirable, was evaluated with a log-rank test. Clinical decisions not to extubate were used as censor points, and were as follows: time of restarted infusions, time of death (i.e. withdrawal of care) or the time limit of 48 h of observation. A traditional analysis of the time to extubation was also performed.
Where quantile–quantile plots indicated a normal distribution, group comparisons were done with Student’s t test. Comparisons for non-normally distributed data and circulatory variables were performed using the Mann–Whitney U test. Dichotomous data were analysed with the unconditional z-pooled test [22]. Two-sided p values less than 0.05 were considered significant.
The log-rank test was performed in PASW Statistics version 17.0.2. Other statistical calculations were performed using R version 2.12.1, with the packages chron, foreign, exactRankTests and an implementation of the unconditional z-pooled test [23, 24].
Ethics
The regional ethics committee approved the study. The attending physician approved participation. Next of kin were informed as soon as possible, and allowed to withdraw the patient from the study at any time. All participants with a good outcome gave deferred informed consent.
Results
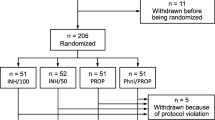
Seventy-four patients were evaluated from April 2008 to May 2009. Sixty patients were included, randomised and given the allocated treatment (Fig. 1). One patient was withdrawn from the study by the next of kin. Thus, 29 and 30 patients participated in the PR and MF group, respectively.
Patient characteristics and Utstein data were similar in both groups (Table 1). Clinical variables at inclusion were similar in both groups, except for a lower first recorded MAP in the PR group (Table 2). The initial doses of the study drugs [median (range)] were propofol 1.5 (0.05–6) mg/kg/h, remifentanil 0.1 (0.05–0.2) μg/kg/min, midazolam 5 (2.5–15) mg/h and fentanyl 0.1 (0.05–0.5) mg/h. For the entire infusion, mean ± SD doses were 2.78 ± 0.8 mg/kg/h propofol, 0.15 ± 0.06 μg/kg/min remifentanil, 13.4 ± 4.8 mg/h midazolam and 0.2 ± 0.08 mg/h fentanyl. Neuromuscular blocking agents were used in 17 PR and 19 MF patients, respectively (p = 0.81).
Time from discontinuation to either extubation or decision not to extubate was significantly lower in the PR group compared with the MF group [mean (95 % confidence intervals) 13.2 (2.3–24) vs. 36.8 (28.5–45.1) h, respectively, p < 0.001, Fig. 2]. For those extubated, the time to extubation was significantly lower in the PR group (median (range) 0.25 (0–0.72) vs. 12.7 (0.5–26.4) h, p = 0.002). Further need for sedation prevented discontinuation of study drugs according to protocol in 24 patients (11 PR and 13 MF patients, respectively).
All PR patients and 27 of 30 MF patients received vasoactive medications. Detailed circulatory variables were analysed for 27 PR and 23 MF patients, respectively. Two PR and 7 MF patients were not analysed because of a short duration of study drug infusions. In three of these (2 PR and 1 MF patient), study infusions were discontinued shortly after allocation because of circulatory instability. Medical records of circulatory variables were lost for one participant. Circulatory variables, including need for supportive treatment, were similar except that more PR patients required an infusion of norepinephrine (23 vs. 12 patients, respectively, p = 0.003, Table 3). Assigning worse than all actual observed values to the patients removed because of circulatory instability did not alter the results.
The incidences of pneumonia and VAP were similar in both groups (Table 4). Fourteen and 8 patients had a good 3-month outcome in the PR and MF groups, respectively (p = 0.11). Six of the 24 patients where discontinuation could not be performed in the 72-h study observation time recovered with a good outcome.
Discussion
In this randomised, controlled trial, sedation and analgesia with PR resulted in a faster offset of effect defined as the time to extubation or decision not to extubate after TH versus sedation with MF. The time to extubation was below 1 h in all PR patients, and varied from 0.5 to 26 h in the MF patients. The PR patients needed administration of norepinephrine twice as often as MF patients, but circulatory variables were otherwise similar. The protocols were tolerated in 56 of 59 participants.
Our finding of a faster offset in the PR group agrees with previous studies on other ICU patients. Muellejans et al. [19] reported a shorter time to extubation with remifentanil and additional propofol than with midazolam and fentanyl (2.2 vs. 5.7 h, respectively). Bauer et al. [20] reported a mean time to extubation of 0.8 and 8 h in patients given PR and MF, respectively. A change from different combinations of flunitrazepam, midazolam, sufentanil and fentanyl to propofol and remifentanil was also associated with a shorter time to extubation [25]. In contrast to Rozendaal et al. [26], Spies et al. [27] reported no difference in weaning time.
The differences in times to offset for both analyses of the primary endpoint were large. First, this may be explained by the accumulation of sedatives and/or analgesics due to decreased metabolism because of hypothermia [28]. In pigs, concentrations of fentanyl increase by 25 % during mild hypothermia [29]. Population pharmacokinetic modelling of data from human volunteers predicted an 11.1 % decrease in midazolam clearance for each degree Celsius reduction in core temperature [30]. In patients with traumatic brain injuries, serum concentrations of midazolam were elevated during TH [31]. Elimination of propofol and remifentanil is decreased during hypothermia, but their short duration of action reduce the potential increase in the time to extubation [32, 33]. Second, the patients may be given higher doses of sedatives and analgesics than needed [34], especially during neuromuscular blockade because of concerns of awareness. The impact on extubation time from a relative overdose of analgesics or sedatives is likely larger for drugs with a longer duration of action.
The time to offset was shorter in PR patients. However, in 24 patients discontinuation of sedation and analgesia could not be performed because of respiratory complications, circulatory instability or early death. A fast pharmacological offset of action does not translate into a clinical benefit when discontinuation is undesirable because of seizures or respiratory failure. Thus, about half the patients in this study would not clinically benefit from a fast-offset protocol. For these, the actual considerations for choice of sedation and analgesia are limited to effect on seizures, adverse effects and drug costs.
Compromised circulation is associated with poor outcome, and the risk is considered higher for PR than for MF [35–38]. In this study, the first registered MAP was significantly lower, and infusions of norepinephrine were required twice as often in the PR group. No differences were observed in other markers of circulatory function such as fluid administration, lactate serum concentration and arterial blood pH. This suggests that a low blood pressure during initiation of ICU treatment and study drugs was subsequently balanced with vasoactive agents. A secondary analysis of circulatory variables, where the 3 patients (2 PR, 1 MF) removed from study because of circulatory instability were assigned worse than all observed values, did not change these findings. This suggests that if circulation is adequately monitored and supported with therapeutic interventions, both protocols can be used safely in most patients treated with TH following CA.
Infection is a clinical concern during TH, but there was no difference in the incidence of VAP [39, 40]. Both groups were subject to standardised routines for withdrawal of care, and there was no difference in CPC scores after 3 months. However, the study was not powered for these endpoints, which only indicate that no unexpected large differences exist with regard to VAP or neurological outcome.
We recognize some strengths and limitations of this study. Pragmatic study procedures were chosen to adhere to the standard TH treatment protocol to increase external validity [41]. The participating hospitals were both regional centres for TH treatment which enabled screening of the entire TH population in the respective catchment areas. All patients treated with TH after CA were assessed and owing to non-strict selection criteria, most patients were included (60 of 74 screened patients). This argues for the generalisability of the findings of this study, which was performed without funding or support from pharmaceutical companies.
A potential limitation of the study was its open design. MF and PR differ both visually and with regards to administration protocols. A double dummy procedure was considered unfeasible in the clinical setting of TH. However, predefined criteria for extubation were used, and the potential time frame in which a clinician could potentially influence the time to extubation by using pharmacodynamic knowledge of the two protocols cannot explain a median difference of 12 h. Second, in other studies on the offset from sedation and analgesia, the time to extubation is the usual endpoint. In the present study 17 patients were extubated according to study protocol, illustrating that in this population pneumonia and post-anoxic cerebral pathology often prevent discontinuation of sedation and extubation. Therefore, the time to offset was defined as the time to extubation or decision not to extubate. Extubation, restarting infusions for sedation and analgesia, death or a timeout of 48 h are all distinct events. Thus, although the analysis of the time to offset is uncommon, we believe it is valid and appropriate in patients treated with TH. Third, the lack of a standardised tool validated in Norwegian to assess pain in intubated ICU patients prevented us from screening for pain. Fourth, sedatives and analgesics administered as fractional doses before allocation could theoretically increase the time to offset. However, randomisation would distribute the use of medications before inclusion evenly between the groups. All PR extubations were performed within 1 h, indicating that there were no residual effects from these medications. Fifth, 10 patients were excluded because of imminent circulatory shock, recurrent cardiac arrests and/or poor prognosis. This study can therefore not document the suitability of PR or MF in patients with severe cardiovascular instability. Sixth, the use of electroencephalographic monitoring during TH has recently been proposed in order to detect epileptogenic activity [39]. Midazolam and propofol are potent inhibitors of seizures, but because electroencephalographic monitoring was not performed, this study does not compare the effects from these drugs on subclinical epileptogenic activity. Seventh, the choice of 4 h as a minimally clinically important difference could be questioned. However, a difference larger than 4 h often implies that patients are not extubated during the ordinary daytime shifts. This may result in an additional day with mechanical ventilation because procedures such as extubation are often postponed to the next morning because of staff limitations at night. Finally, this study is not a direct comparison of two different opioids or two different sedatives, but of two different drug combinations—the currently most common protocol for sedation and analgesia during TH and a protocol using drugs with known fast offset of action.
Conclusions
The time from discontinuation to offset of sedation and analgesia is significantly shorter in patients sedated with PR compared to MF. However, the clinical course in 40 % of patients prevented discontinuation and potential benefits from a faster recovery. The PR group required norepinephrine twice as often, but both protocols were tolerated in most patients.
Abbreviations
- ALT:
-
Alanine aminotransferase
- CA:
-
Cardiac arrest
- CCU:
-
Coronary care unit
- CPC:
-
Cerebral performance category
- GCS:
-
Glasgow coma scale
- CPIS:
-
Clinical pulmonary infection score
- ICU:
-
Intensive care unit
- MAAS:
-
Motor activity assessment scale
- MAP:
-
Mean arterial pressure
- MF:
-
Midazolam and fentanyl
- PR:
-
Propofol and remifentanil
- PT-INR:
-
International normalised ratio of prothrombin time
- SAPS II:
-
Simplified acute physiology score II
- SD:
-
Standard deviation
- TH:
-
Therapeutic hypothermia
- VAP:
-
Ventilator-associated pneumonia
References
Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K (2002) Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 346:557–563
The Hypothermia After Cardiac Arrest Study Group (2002) Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 346:549–556
Neumar RW, Nolan JP, Adrie C et al (2008) Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation 118:2452–2483
Chamorro C, Borrallo JM, Romera MA, Silva JA, Balandín B (2010) Anesthesia and analgesia protocol during therapeutic hypothermia after cardiac arrest: a systematic review. Anesth Analg 110:1328–1335
Varghese JM, Roberts JA, Lipman J (2010) Pharmacokinetics and pharmacodynamics in critically ill patients. Curr Opin Anaesthesiol 23:472–478
Tortorici MA, Kochanek PM, Poloyac SM (2007) Effects of hypothermia on drug disposition, metabolism, and response: a focus of hypothermia-mediated alterations on the cytochrome P450 enzyme system. Crit Care Med 35:2196–2204
Mortensen B, Dale O (1995) Effects of hypothermia on the elimination of ethanol, diazepam and oxazepam in rat liver slice incubations. Acta Anaesthesiol Scand 39:199–204
Polderman KH (2004) Application of therapeutic hypothermia in the ICU: opportunities and pitfalls of a promising treatment modality. Part 1: indications and evidence. Intensive Care Med 30:556–575
Alcaraz C, Bansinath M, Turndorf H, Puig MM (1989) Cardiovascular effects of morphine during hypothermia. Arch Int Pharmacodyn Ther 297:133–147
Vincent JL, Moreno R, Takala J, Willatts S, De Mendona A, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
Devlin JW, Boleski G, Mlynarek M, Nerenz DR, Peterson E, Jankowski M, Horst HM, Zarowitz BJ (1999) Motor activity assessment scale: a valid and reliable sedation scale for use with mechanically ventilated patients in an adult surgical intensive care unit. Crit Care Med 27:1271–1275
Cummins RO, Chamberlain DA, Abramson NS et al (1991) Recommended guidelines for uniform reporting of data from out-of-hospital cardiac arrest: the Utstein style. A statement for health professionals from a task force of the American Heart Association, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, and the Australian Resuscitation Council. Circulation 84:960–975
Le Gall JR, Lemeshow S, Saulnier F (1993) A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Teasdale G, Jennett B (1974) Assessment of coma and impaired consciousness: a practical scale. Lancet 2:81–84
Luna CM, Blanzaco D, Niederman MS et al (2003) Resolution of ventilator-associated pneumonia: prospective evaluation of the clinical pulmonary infection score as an early clinical predictor of outcome. Crit Care Med 31:676–682
Wijdicks EF, Hijdra A, Young GB, Bassetti CL, Wiebe S (2006) Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 67:203–210
Jennett B, Bond M (1975) Assessment of outcome after severe brain damage. Lancet 1:480–484
Brain Resuscitation Clinical Trial I Study Group (1986) A randomized clinical study of cardiopulmonary-cerebral resuscitation: design, methods, and patient characteristics. Brain Resuscitation Clinical Trial I Study Group. Am J Emerg Med 4:72–86
Muellejans B, Matthey T, Scholpp J, Schill M (2006) Sedation in the intensive care unit with remifentanil/propofol versus midazolam/fentanyl: a randomised, open-label, pharmacoeconomic trial. Crit Care 10:R91
Bauer C, Kreuer S, Ketter R, Grundmann U, Wilhelm W (2007) Remifentanil-propofol versus fentanyl-midazolam combinations for intracranial surgery: influence of anaesthesia technique and intensive sedation on ventilation times and duration of stay in the ICU. Der Anaesthesist 56:128–132
Carrasco G, Molina R, Costa J, Soler JM, Cabré L (1993) Propofol versus midazolam in short-, medium-, and long-term sedation of critically ill patients: a cost-benefit analysis. Chest 103:557–564
Lydersen S, Fagerland MW, Laake P (2009) Recommended tests for association in 2 × 2 tables. Stat Med 28:1159–1175
R Development Core Team (2008) R: A language and environment for statistical computing. R foundation for statistical computing. http://www.R-project.org. Accessed 18 Feb 2011
Galili T (2010) Barnard’s exact test—a powerful alternative for Fisher’s exact test (implemented in R). http://www.r-statistics.com/2010/02/barnards-exact-test-a-powerful-alternative-for-fishers-exact-test-implemented-in-r/. Accessed 13 Feb 2011
Muller L, Chanques G, Bourgaux C, Louart G, Jaber S, Fabbro-Peray P, Ripart J, de La Coussaye JE, Lefrant JY (2008) Impact of the use of propofol remifentanil goal-directed sedation adapted by nurses on the time to extubation in mechanically ventilated ICU patients: the experience of a French ICU. Ann Fr Anesth Reanim 27:481.e1–481.e8
Rozendaal FW, Spronk PE, Snellen FF, Schoen A, van Zanten AR, Foudraine NA, Mulder PG, Bakker J (2009) Remifentanil-propofol analgo-sedation shortens duration of ventilation and length of ICU stay compared to a conventional regimen: a centre randomised, cross-over, open-label study in the Netherlands. Intensive Care Med 35:291–298
Spies C, MacGuill M, Heymann A et al (2011) A prospective, randomized, double-blind, multicenter study comparing remifentanil with fentanyl in mechanically ventilated patients. Intensive Care Med 37:469–476
Arpino PA, Greer DM (2008) Practical pharmacologic aspects of therapeutic hypothermia after cardiac arrest. Pharmacotherapy 28:102–111
Fritz HG, Holzmayr M, Walter B, Moeritz KU, Lupp A, Bauer R (2005) The effect of mild hypothermia on plasma fentanyl concentration and biotransformation in juvenile pigs. Anesth Analg 100:996–1002
Hostler D, Zhou J, Tortorici MA, Bies RR, Rittenberger JC, Empey PE, Kochanek PM, Callaway CW, Poloyac SM (2010) Mild hypothermia alters midazolam pharmacokinetics in normal healthy volunteers. Drug Metab Dispos 38:781–788
Fukuoka N, Aibiki M, Tsukamoto T, Seki K, Morita S (2004) Biphasic concentration change during continuous midazolam administration in brain-injured patients undergoing therapeutic moderate hypothermia. Resuscitation 60:225–230
Michelsen LG, Holford NH, Lu W, Hoke JF, Hug CC, Bailey JM (2001) The pharmacokinetics of remifentanil in patients undergoing coronary artery bypass grafting with cardiopulmonary bypass. Anesth Analg 93:1100–1105
Leslie K, Sessler DI, Bjorksten AR, Moayeri A (1995) Mild hypothermia alters propofol pharmacokinetics and increases the duration of action of atracurium. Anesth Analg 80:1007–1014
Payen JF, Chanques G, Mantz J et al (2007) Current practices in sedation and analgesia for mechanically ventilated critically ill patients: a prospective multicenter patient-based study. Anesthesiology 106:687–695
Sebel PS, Hoke JF, Westmoreland C, Hug CC, Muir KT, Szlam F (1995) Histamine concentrations and hemodynamic responses after remifentanil. Anesth Analg 80:990–993
Shafer A (1998) Complications of sedation with midazolam in the intensive care unit and a comparison with other sedative regimens. Crit Care Med 26:947–956
Komatsu R, Turan AM, Orhan-Sungur M, McGuire J, Radke OC, Apfel CC (2007) Remifentanil for general anaesthesia: a systematic review. Anaesthesia 62:1266–1280
Marchick MR, Kline JA, Jones AE (2009) The significance of non-sustained hypotension in emergency department patients with sepsis. Intensive Care Med 35:1261–1264
Nielsen N, Sunde K, Hovdenes J, Riker RR, Rubertsson S, Stammet P, Nilsson F, Friberg H (2011) Adverse events and their relation to mortality in out-of-hospital cardiac arrest patients treated with therapeutic hypothermia. Crit Care Med 39:57–64
Mongardon N, Perbet S, Lemiale V et al (2011) Infectious complications in out-of-hospital cardiac arrest patients in the therapeutic hypothermia era. Crit Care Med 39:1359–1364
Zwarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S, Haynes B, Oxman AD, Moher D (2008) Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 337:a2390
Acknowledgments
We thank Professor Eldar Søreide MD, PhD for facilitating the trial. We also thank nurses and physicians at the three units for assistance with inclusions and data collection. The Unit for Applied Clinical Research, Norwegian University of Science and Technology, developed and provided the Web-based interface and computerized randomisation.
Conflicts of interest
PK and KK received support for travel to a scientific meeting. No other authors have declared potential conflicts of interest. This study was funded by the Norwegian University of Science and Technology. Grants for sub-studies were given by St. Olavs Hospital and The Royal Norwegian Society of Sciences and Letters. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial registration: ClinicalTrials.gov NCT00667043
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bjelland, T.W., Dale, O., Kaisen, K. et al. Propofol and remifentanil versus midazolam and fentanyl for sedation during therapeutic hypothermia after cardiac arrest: a randomised trial. Intensive Care Med 38, 959–967 (2012). https://doi.org/10.1007/s00134-012-2540-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2540-1