Abstract
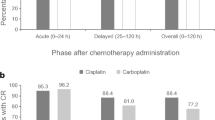
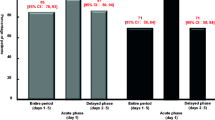
The clinical efficacy and tolerability of a new nasal spray formulation of metoclopramide (MTC) was evaluated in terms of its ability to prevent the nausea and vomiting induced by a moderately emetic chemotherapy (cisplatin 20 mg/m2 weekly as radioenhancer+radiotherapy for a fractionated total of 60 Gy) in 12 patients with non-small-cell lung cancer, stage IIIB. The first chemotherapy cycle was administered without any prophylaxis in order to identify those patients who experienced grade 2 nausea and/or vomiting. As prophylaxis during the second cycle, these patients were given MTC 20 mg i.v. at time zero, and MTC 20 mg i.m. after 4 h and 8 h; during the third cycle, they received MTC 40 mg by nasal spray 2 h before chemotherapy, followed by the same dose at 4 h and 8 h. The two prophylactic treatments (parenteral injections and nasal spray) proved to be therapeutically equivalent: complete protection, 6 and 6 patients respectively; major protection, 2 and 3 patients; minor protection, 1 and 1 patients; no protection, 3 and 2 patients. The control of nausea was satisfactory, with 7 and 9 patients respectively experiencing grade 0–1 nausea. Comparative analysis of individual responses confirmed the similar anti-emetic efficacy of the two regimens. No adverse reactions were observed at any time during the course of the study, and all 12 patients judged the acceptability of the new formulation as optimal. It can thus be concluded that the use of metoclopramide nasal spray represents an effective, safe, easily managed and low-cost therapeutic alternative for the prophylaxis and treatment of emesis induced by low-dose chemotherapy. The results of the present study could also promote a regular use of the MTC nasal spray in other fields of oncological supportive therapy.
Similar content being viewed by others
References
Desmond PV, Watson KJR (1986) Metoclopramide — a review. Med J Aust 144:366–369
Du Bois A, Meerpohl HG, Vach V, et al (1992) Course, patterns and risk factors for chemotherapy-induced emesis in cisplatin-pretreated patients: a study with ondansetron. Eur J Cancer 28:450–457
Powis G, Macker MP (1991) The toxicity of anticancer drugs. Pergamon Press, New York
Guslandi M (1989) Drugs for upper digestive motility disorders. Drugs Today 25:101–110
Mc Dermed J, Cohen J, Huang C, et al (1985) Correlative data relating serum metoclopramide levels and serum cisplantin levels: preliminary results. Proc Am Soc Clin Oncol 4:261
Mitchelson F (1992) Pharmacological agents affecting emesis. A review. Drugs 43:295–315
Scaglion F, Scanni A, Tomirotti M, et al (1993) Pharmacokinetics and bioavailability of metoclopramide nasal spray vs metoclopramide intravenous in helathy volunteers and cancer patients. Arzneimittelforschung/Drug Res 43:986–991
Seynaeve C, De Mulder PHM, Verweij J, Gralla RJ (1991) Controlling cancer chemotherapy-induced emesis. An update. Pharm Weekbl Sci 13:189–197
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tomirotti, M., Dimaiuta, M., Confalonieri, M. et al. Efficacy and tolerability of nasally administered compared to parenterally administered metoclopramide in the symptomatic treatment of chemotherapy-induced emesis in cancer outpatients A controlled clinical study. Support Care Cancer 2, 389–392 (1994). https://doi.org/10.1007/BF00344054
Issue Date:
DOI: https://doi.org/10.1007/BF00344054