Abstract
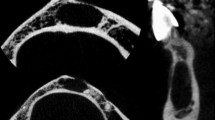
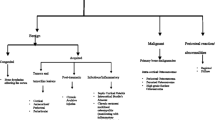
The periosteum covers most bone structures. It has an outer fibrous layer and an inner cambial layer that exhibits osteogenic activity. The periosteum is a dynamic structure that plays a major role in bone modeling and remodeling under normal conditions. In several disorders such as infections, benign and malignant tumors, and systemic diseases, the osteogenic potential of the periosteum is stimulated and new bone is produced. The newly formed bone added onto the surface of the cortex adopts various configurations depending on the modalities and pace of bone production. Our aim here is to describe the anatomy, histology, and physiology of the periosteum and to review the various patterns of periosteal reaction with emphasis on relations between radiological and histopathological findings. A careful evaluation of the periosteal reaction and appearance of the underlying cortex, in combination with the MRI, clinical, and laboratory data, provides valuable information on lesion duration and aggressiveness, thereby assisting in the etiological diagnosis and optimizing patient management. A solid reaction strongly suggests a benign and slow-growing process that gives the bone enough time to wall off the lesion. Single lamellar reactions occur in acute and usually benign diseases. Multilamellar reactions are associated with intermediate aggressiveness and a growth rate close to the limit of the walling-off capabilities of the bone. Spiculated, interrupted, and complex combined reactions carry the worst prognosis, as they occur in the most aggressive and fast-growing diseases: the periosteum attempts to create new bone but is overwhelmed and may be breached.























Similar content being viewed by others
References
Dwek JR. The periosteum: what is it, where is it, and what mimics it in its absence? Skeletal Radiol. 2010;39(4):319–23.
Rauch F. Bone growth in length and width: the Yin and Yang of bone stability. J Musculoskelet Neuronal Interact. 2005;5(3):194–201.
Orwoll ES. Toward an expanded understanding of the role of the periosteum in skeletal health. J Bone Miner Res. 2003;18(6):949–54.
Zhang X, Awad HA, O’Keefe RJ, Guldberg RE, Schwarz EM. A perspective: engineering periosteum for structural bone graft healing. Clin Orthop Relat Res. 2008;466(8):1777–87.
Allen MR, Hock JM, Burr DB. Periosteum: biology, regulation, and response to osteoporosis therapies. Bone. 2004;35(5):1003–12.
Ragsdale BD, Madewell JE, Sweet DE. Radiologic and pathologic analysis of solitary bone lesions. Part II: periosteal reactions. Radiol Clin North Am. 1981;19(4):749–83.
Mills SE, editor. Histology for pathologists. 4th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012.
Klein MJ. Non-Neoplastic Diseases of Bones and Joints: Atlas of Nontumor Pathology. American Registry of Pathology; 2011.
Hill EL, Elde R. Distribution of CGRP-, VIP-, D beta H-, SP-, and NPY-immunoreactive nerves in the periosteum of the rat. Cell Tissue Res. 1991;264(3):469–80.
Hohmann EL, Elde RP, Rysavy JA, Einzig S, Gebhard RL. Innervation of periosteum and bone by sympathetic vasoactive intestinal peptide-containing nerve fibers. Science. 1986;232(4752):868–71.
Mach DB, Rogers SD, Sabino MC, Luger NM, Schwei MJ, Pomonis JD, et al. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113(1):155–66.
Tonna EA. Response of the cellular phase of the skeleton to trauma. Periodontics. 1966;4(3):105–14.
Tang XM, Chai BF. Ultrastructural investigation of osteogenic cells. Chin Med J. 1986;99(12):950–6.
Squier CA, Ghoneim S, Kremenak CR. Ultrastructure of the periosteum from membrane bone. J Anat. 1990;171:233–9.
Ito Y, Fitzsimmons JS, Sanyal A, Mello MA, Mukherjee N, O’Driscoll SW. Localization of chondrocyte precursors in periosteum. Osteoarthr Cartil. 2001;9(3):215–23.
Diaz-Flores L, Gutierrez R, Lopez-Alonso A, Gonzalez R, Varela H. Pericytes as a supplementary source of osteoblasts in periosteal osteogenesis. Clin Orthop Relat Res. 1992;275:280–6.
Duhamel H. Cited by Bassett CAL in current concepts of bone formation. J Bone Joint Surg. 1739;44-A:1217–44.
Ollier L. Traité expérimentale et clinique de la régénération des os et de la production artificielle du tissu osseux. Paris: Masson et Fils; 1867.
Jacobsen FS. Periosteum: its relation to pediatric fractures. J Pediatr Orthop B. 1997;6(2):84–90.
Szulc P, Seeman E, Duboeuf F, Sornay-Rendu E, Delmas PD. Bone fragility: failure of periosteal apposition to compensate for increased endocortical resorption in postmenopausal women. J Bone Miner Res. 2006;21(12):1856–63.
Seeman E. Periosteal bone formation–a neglected determinant of bone strength. N Engl J Med. 2003;349(4):320–3.
Kim B-T, Mosekilde L, Duan Y, Zhang X-Z, Tornvig L, Thomsen JS, et al. The structural and hormonal basis of sex differences in peak appendicular bone strength in rats. J Bone Miner Res. 2003;18(1):150–5.
Turner RT, Wakley GK, Hannon KS. Differential effects of androgens on cortical bone histomorphometry in gonadectomized male and female rats. J Orthop Res. 1990;8(4):612–7.
Parfitt AM. Parathyroid hormone and periosteal bone expansion. J Bone Miner Res. 2002;17(10):1741–3.
Dempster DW, Cosman F, Kurland ES, Zhou H, Nieves J, Woelfert L, et al. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res. 2001;16(10):1846–53.
Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, Eriksen EF. Recombinant human parathyroid hormone (1-34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res. 2003;18(11):1932–41.
Ma YL, Zeng Q, Donley DW, Ste-Marie L-G, Gallagher JC, Dalsky GP, et al. Teriparatide increases bone formation in modeling and remodeling osteons and enhances IGF-II immunoreactivity in postmenopausal women with osteoporosis. J Bone Miner Res. 2006;21(6):855–64.
Specker B, Binkley T. Randomized trial of physical activity and calcium supplementation on bone mineral content in 3- to 5-year-old children. J Bone Miner Res. 2003;18(5):885–92.
Zhu K, Greenfield H, Du X, Zhang Q, Fraser DR. Effects of milk supplementation on cortical bone gain in Chinese girls aged 10-12 years. Asia Pac J Clin Nutr. 2003;12:S47.
McKenzie JA, Silva MJ. Comparing histological, vascular and molecular responses associated with woven and lamellar bone formation induced by mechanical loading in the rat ulna. Bone. 2011;48(2):250–8.
Feik SA, Ellender G, Crowe DM, Ramm-Anderson SM. Periosteal response in translation-induced bone remodelling. J Anat. 1990;171:69–84.
Frost HM, Schönau E. The “muscle-bone unit” in children and adolescents: a 2000 overview. J Pediatr Endocrinol Metab. 2000;13(6):571–90.
Hamrick MW, McNeil PL, Patterson SL. Role of muscle-derived growth factors in bone formation. J Musculoskelet Neuronal Interact. 2010;10(1):64–70.
Elkasrawy MN, Hamrick MW. Myostatin (GDF-8) as a key factor linking muscle mass and bone structure. J Musculoskelet Neuronal Interact. 2010;10(1):56–63.
Simpson AH. The blood supply of the periosteum. J Anat. 1985;140(Pt 4):697–704.
Kenan S, Abdelwahab IF, Klein MJ, Hermann G, Lewis MM. Lesions of juxtacortical origin (surface lesions of bone). Skeletal Radiol. 1993;22(5):337–57.
Wenaden AET, Szyszko TA, Saifuddin A. Imaging of periosteal reactions associated with focal lesions of bone. Clin Radiol. 2005;60(4):439–56.
Rana RS, Wu JS, Eisenberg RL. Periosteal reaction. AJR Am J Roentgenol. 2009;193(4):W259–272.
Miller TT. Bone tumors and tumorlike conditions: analysis with conventional radiography. Radiology. 2008;246(3):662–74.
Ballikar R, Balikar R, Redkar NN, Patil MA, Pillai R. Hair-on-end appearance in a case of thalassemia intermedia. BMJ Case Rep. 2013;2013.
Bastug D, Ortiz O, Schochet SS. Hemangiomas in the calvaria: imaging findings. AJR Am J Roentgenol. 1995;164(3):683–7.
Kim KS, Rogers LF, Goldblatt D. CT features of hyperostosing meningioma en plaque. AJR Am J Roentgenol. 1987;149(5):1017–23.
Sundaram M, McGuire MH. Computed tomography or magnetic resonance for evaluating the solitary tumor or tumor-like lesion of bone? Skeletal Radiol. 1988;17(6):393–401.
Magid D. Two-dimensional and three-dimensional computed tomographic imaging in musculoskeletal tumors. Radiol Clin North Am. 1993;31(2):425–47.
Greenfield GB, Warren DL, Clark RA. MR imaging of periosteal and cortical changes of bone. Radiographics. 1991;11(4):611–23. discussion 624.
Dosdá R, Martí-Bonmatí L, Menor F, Aparisi F, Rodrigo C, Ricart V. Comparison of plain radiographs and magnetic resonance images in the evaluation of periosteal reaction and osteoid matrix in osteosarcomas. MAGMA. 1999;9(1–2):72–80.
Spaeth HJ, Chandnani VP, Beltran J, Lucas JG, Ortiz I, King MA, et al. Magnetic resonance imaging detection of early experimental periostitis. Comparison of magnetic resonance imaging, computed tomography, and plain radiography with histopathologic correlation. Invest Radiol. 1991;26(4):304–8.
Bloem JL, Taminiau AH, Eulderink F, Hermans J, Pauwels EK. Radiologic staging of primary bone sarcoma: MR imaging, scintigraphy, angiography, and CT correlated with pathologic examination. Radiology. 1988;169(3):805–10.
Frouge C, Vanel D, Coffre C, Couanet D, Contesso G, Sarrazin D. The role of magnetic resonance imaging in the evaluation of Ewing sarcoma. A report of 27 cases. Skeletal Radiol. 1988;17(6):387–92.
Saifuddin A. The accuracy of imaging in the local staging of appendicular osteosarcoma. Skeletal Radiol. 2002;31(4):191–201.
Saifuddin A, Burnett SJ, Mitchell R. Pictorial review: ultrasonography of primary bone tumours. Clin Radiol. 1998;53(4):239–46.
Bilkay U, Tokat C, Helvaci E, Ozek C, Zekioglu O, Onat T, et al. Osteogenic capacities of tibial and cranial periosteum: a biochemical and histologic study. J Craniofac Surg. 2008;19(2):453–8.
Lodwick G. A systematic approach to the roentgen diagnosis of bone tumors. Tumors of bone and soft tissue. M.D. Anderson Hospital and Tumor Institute, Chicago; 1965. p. 49–68.
Lodwick GS, Wilson AJ, Farrell C, Virtama P, Dittrich F. Determining growth rates of focal lesions of bone from radiographs. Radiology. 1980;134(3):577–83.
Madewell JE, Ragsdale BD, Sweet DE. Radiologic and pathologic analysis of solitary bone lesions. Part I: internal margins. Radiol Clin North Am. 1981;19(4):715–48.
Conflict of interest
None of the authors have any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bisseret, D., Kaci, R., Lafage-Proust, MH. et al. Periosteum: Characteristic imaging findings with emphasis on radiologic-pathologic comparisons. Skeletal Radiol 44, 321–338 (2015). https://doi.org/10.1007/s00256-014-1976-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-014-1976-5